NHC-based Palladium Catalysts
In collaboration with Umicore AG and Co.,1 we are offering a series of robust Pd(II) and Pd(0) complexes used as the linchpin in C–C bond forming reactions. The high-performance Pd catalysts can efficiently couple alkyl- and aryl chlorides with organoboron compounds on large scale (100 g–100 t).2 The high TONs, mild reaction conditions, and economic viability/availability of aryl chlorides, make this methodology attractive to industrial scale applications. Catalysts 1 and 2 exhibit superior activity in C–C coupling reactions. They are formally Pd(0) and are rare examples of well-characterized monocarbene palladium precursors to 12-electron complexes. The Umicore NHC-Pd system performs Suzuki and Kumada couplings as well as α-arylation reactions at reasonable temperatures.
In the latter case [(NHC)Pd(allyl)Cl],2 a reactive, formally 16- electron complex, mediates the α-arylation of an array of aryl ketones (Scheme 1).3 The air-stable catalyst, short reaction times, and high conversions prove the usefulness of this NHC technology over previous Pd catalyzed α-arylations. The reactivity of both alkyl–alkyl and alkyl–aryl ketones was studied in this early NHC-Pd article from Nolan and co-workers.
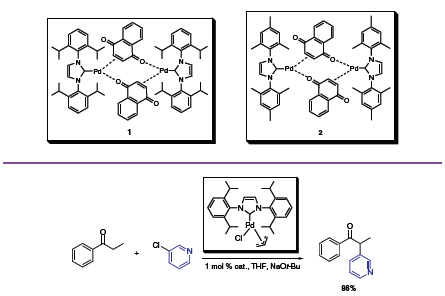
Scheme 1. α-arylation of an array of aryl ketones
[{Pd(IMes)(NQ)}2] catalyst 2 demonstrated high reactivity and selectivity in sp3–sp2 Kumada couplings (Scheme 2).4 The generality of this methodology extends to both electron-rich and electron-poor arylmagnesium reagents. Furthermore, a broad spectrum of functionalized alkyl chlorides were used to afford complex organic building blocks. The high product yields at room temperature validates the robustness of the catalytic system versus well-known Pd-phosphine catalysts (Pd(PPh3)4, Pd2dba3) as a function of reaction conditions.
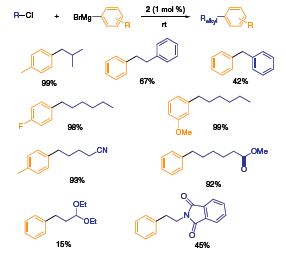
Scheme 2.Kumada couplings
The related [{Pd(IPr)(NQ)}2] catalyst 1 exhibited impressive activity in the Suzuki-Miyaura coupling of aryl chlorides with phenyl boronic acids (Scheme 3). At 50 °C, the high-yielding (88%) reaction was complete in one hour at a catalyst loading of 0.5 mol %.5 Interestingly, Pd(0) catalyst 1 produced lower yields of coupled biaryl product at room temperature, whereas analogous catalyst 2 gave 86% yields of 4-Cl-biphenyl at both room temperature and 50 °C under identical conditions. Presumably, catalyst 1 needs additional energy to climb over the activation barrier and enter the catalytic cycle as a naked Pd-NHC species. It should be noted that the reactivity of [{Pd(IPr)(NQ)}2] was also shown to be high in the coupling of sterically encumbered 2,6-diphenyl chloride and 1-naphthaleneboronic acid.
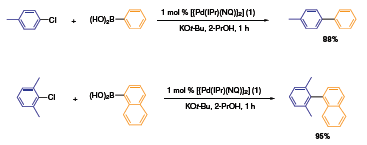
Scheme 3. Suzuki-Miyaura coupling of aryl chlorides
Beller and co-workers established a reactivity profile for NHC-Pd naphthoquinone catalysts in Heck reactions (Table 1).6 The outstanding capacity of this system is illustrated in the following scheme, wherein the best stilbene yields were obtained at 140 °C in an ionic liquid medium. The low catalyst loading (0.5 mol %), cheap aryl chloride reagents, and a stabilized ionic liquid environment contribute to the potential advancement of this chemistry to the industrial fine chemicals arena.
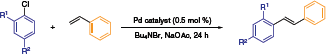
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?