Carreira DOLEFIN Ligands
The selective preparation of diarylmethine stereogenic centers is a challenging endeavor in chemical synthesis, especially when there is little differentiation between the two aryl groups. As a result, the methods for the stereoselective synthesis of this motif are rather limited.1 Recent work by Carreira described the use of chiral dienes derived from [2.2.2]bicyclooctadiene as ligands in various asymmetric processes.2 More recently, Carreira has used the ligand 1 in the 1,4-addition of arylboronic acids to α,β-unsaturated carbonyls.
![Carreira DOLEFIN Ligands [2.2.2]bicyclooctadiene](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/chemistry-and-synthesis/cross-coupling/bicyclooctadiene.gif)
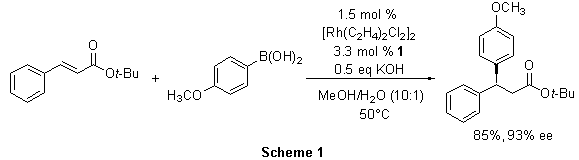
In one report, tert-butyl cinnamate was converted to (S)-tert-butyl 3-(4-methoxyphenyl)-3-phenylpropanoate in the presence of the Rh(I)-1 complex in excellent yield and selectivity (Scheme 1). Similarly, other 3,3-diaryl- and 3-aryl-3-heteroaryl-propanoates were prepared in good to excellent yields and with consistently high enantioselectivity (89-94% ee). Furthermore, from a single enantiomer of the ligand, access to both enantiomers of a product can be achieved simply by switching the aryl acceptor and donor. In this manner, (R)-tert-butyl 3-(4-methoxyphenyl)-3-phenylpropanoate was prepared from phenylboronic acid and tert-butyl 4-methoxycinnamate (Scheme 2).3
-3-phenylpropanoate.gif)
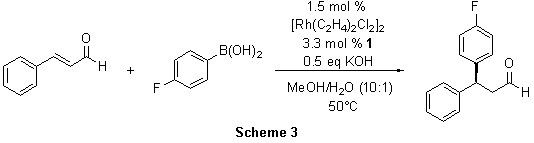
The preparation of chiral 3,3-diarylpropanals has recently been achieved by other researchers via an amine-catalyzed addition of aromatic nucleophiles to 3-substituted acrolein derivatives.4 Unfortunately, this method does not work well with electron poor aromatics. However, the Rh(I)-1-catalyzed conjugate addition offers a general route independent of the electronic nature of the aryl groups. The addition of 4-fluorophenylboronic acid to cinnamaldehyde provided the 1,4-adduct in 90% yield and 93% ee (Scheme 3). Again, other substrates maintained high enantioselectivity in the conjugate addition (89-93% ee).5
we are pleased to offer both enantiomers of this effective ligand for your research purposes.
Product Information
References
如要继续阅读,请登录或创建帐户。
暂无帐户?