Reverse Transfection of Plasmid DNA
Automation is used for many applications to reduce variation caused by manual handling and to obtain reproducible results in high-throughput assays. High-throughput applications, such as knockdown studies or target screenings, often include cell transfection. In the present study, X-tremeGENEHP and X-tremeGENE9 Transfection Reagents (Roche) were tested for their compatibility with an automated transfection workstation, the Biomek FX.
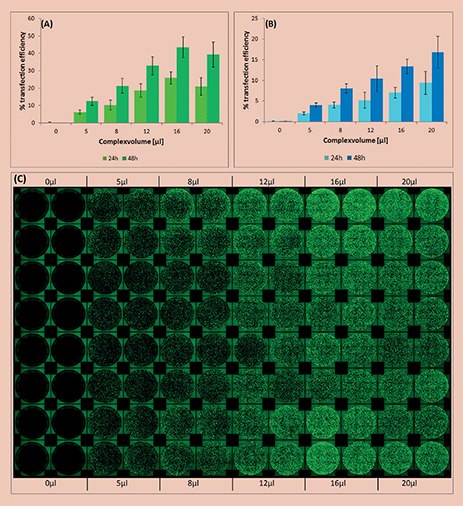
Figure 1.Analysis of transfection efficiency in the automated workflow. The efficiency was measured 24 and 48 hours post transfection and calculated by the ratio of area covered with fluorescing cells compared to total area covered with cells. (A)Transfection using the X-tremeGENEHP Reagent; (B) Transfection using the X-tremeGENE9 Reagent. (C) Top view showing the fluorescence, as well as the reproducibility (16 replicates) of the automated workflow using the X-tremeGENEHP Reagent.
Automated Transfection – Cell seeding and transfection were performed using the Biomek FX (Beckman Coulter) automated workstation. For the transfection, X-tremeGENE HP and X-tremeGENE 9 reagents (Roche) were prediluted in Opti-MEM (dilution factor: 1:50 for X-tremeGENE HP reagent, 1:33 for X-tremeGENE 9 reagent). The EGFP-PC3.1 plasmid was prediluted in Opti-MEM (1 μg/100 μL). The transfectioncomplex was formed by pipetting the same amount of plasmid dilution into the dilution of transfection reagent, followed by mixing and incubation for 30 min.
Cell Culture and Reverse Transfection – HeLa cells (ATCC® CCL-2™) were cultured in MEM with supplements at +37 °C in a 5% CO2 humidified atmosphere in T75 flasks until a confluence of about 70%. Cells were harvested by trypsinization shortly before transfection, washed and resuspended in fresh medium (1.2x105 cells/mL). Reverse transfection was performed by pipetting 100μl cell suspension to each well already containing the transfection complex.
Results
Formation of the transfection complex, cell seeding and transfection were successfully performed in 96-well format using an automated workstation. Increasing volumes of transfection complex from 0 to 20 μL were added to each well, replicates of sixteen for each volume.
The efficiency of transfection was found to be directly dependent on the added volume of transfection complex. As expected, higher transfection efficiency was measured at higher complex volumes until reaching a plateau phase (Figure 1).
Both transfection reagents showed very low cytotoxicity for all volumes tested.
X-tremeGENE HP and 9 Transfection Reagents are fully compatible with automated transfection methods and show a high reproducibility (Figure 1).
Materials
To continue reading please sign in or create an account.
Don't Have An Account?