Synthesis & HILIC/MS Analysis of Acylcarnitines
Introduction
L-Carnitine was discovered at the beginning of the last century and plays an important role in fatty acid metabolic pathways. It acts as a carrier of long chain acyl groups from activated fatty acids across the inner mitochondrial membrane into the mitochondrial matrix where they undergo β-oxidation to acetyl CoA to obtain usable energy via the citric acid cycle.1 The resulting “acyl-L-carnitine” molecule is shown in Figure 1. A number of diseases caused by defects of mitochondrial transport are characterized by specific metabolic dysfunctions and depend on the physiological role of the affected carrier in intermediary metabolism.2 Therefore, the functions and roles of acyl-L-carnitines in various tissues like brain, heart, and muscle continue to attract much interest.3-6
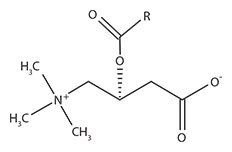
Figure 1.Acyl-L-Carnitine Parent Structure R represents acyl chains of varying length derived from the transported fatty acid.
Analytical Requirements
Newborn screening programs detect treatable disorders in infants before they become symptomatic. Liquid chromatography-tandem mass spectrometry (LC/MS/MS) has greatly increased the screening possibilities in newborn screening programs by monitoring levels of acylcarnitines in order to detect treatable disorders in infants before symptoms appear. LC/MS/MS can detect several disorders with a single injection, which is important in high throughput laboratories. Measuring different acylcarnitines can be used to detect more than 40 different inborn errors of metabolism, a selection of which is shown in Table 1.
Pure and stable metabolite standards as well as robust and sensitive methods for their analysis are prerequisites for investigating the functions of healthy and diseased biological cells on a molecular pathway level. Human metabolic phenotyping in relation to clinical diagnostics, prognostics and epidemiology is therefore one of the most widely applicable areas for the development of precision medicine.7 In order to develop new analytical methods, the synthetic methodologies for the required metabolites need to be established.8
Synthesis of Acyl-L-Carnitines
The expansion of the Sigma-Aldrich portfolio of acyl-L-carnitine standards has been successfully achieved by a generalized synthetic approach. L-Carnitine hydrochloride was added to a mixture of the appropriate carboxylic acid and a slight molar excess of acid chloride. The reaction mixture was kept at an elevated temperature until TLC analysis showed an optimum ratio of the corresponding product to its starting material and side products.9 If the suitable acid chloride was not available, the carboxylic acid was reacted in a first step with thionyl chloride and then the carnitine was added consecutively.
To avoid counter ions (e. g. chloride), which are undesirable in some applications, all acyl-L-carnitines were transformed to their inner salts by a subsequent treatment on a weakly basic anion exchanger.10 After precipitation of the raw product, column chromatography was required to remove any traces of carnitine and side products.
LC/MS Analysis of Acyl-L-Carnitines
Since UV detection of acylcarnitines is very insensitive, a method to allow separation, identification, and quantification with complementary detectors, like mass spectrometry (MS) or charged aerosol detection (CAD), is required. Detection by MS provides the required sensitivity, and direct infusion may be sufficient to analyze a range of different acyl-L-carnitines. However, for more detailed analysis and for measuring acylcarnitine isomers that are closely related, separation by liquid chromatography before detection is important.11,12 Liquid chromatography-mass spectrometry has been established as an efficient and robust methodology for analyzing acyl-L-carnitines and hydrophilic interaction liquid chromatography (HILIC) to separate free carnitine and acylcarnitines is fast and does not need derivatization.13-15 In this study, HILIC mode chromatography was explored using an Ascentis® Express OH5 column with a gradient of acetonitrile in 50 mm ammonium acetate. LC and MS instrument and settings appear in Table 2 and Table 3. Final chromatographic conditions appear in Figure 2.
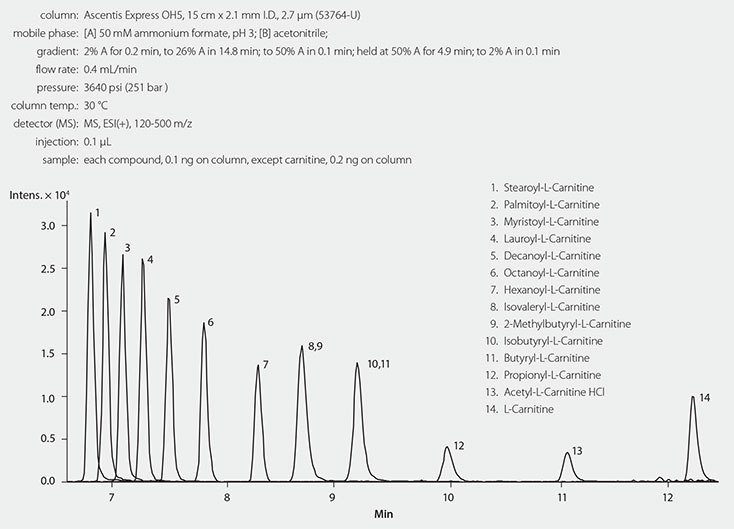
Figure 2.LC/MS Analysis of Twelve Acyl-L-Carnitines on Ascentis Express OH5 (HILIC Mode)
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?