Determination of L- and D-Lactic Acid Enantiomers by HPLC/MS/MS
Introduction
This article summarizes the work of H. Henry and associates at the Clinical Chemistry Laboratory, Centre Hospitalier Universiaire Vaudois, University of Lausanne, CH-1011 Lausanne, Switzerland1. Determination of L- and D-lactic acid enantiomers (Figure 1) and their subsequent quantification is of vital importance in infants, as a major excess of D-lactic acid production can lead to metabolic acidosis resulting into significant neurological symptoms if left untreated. Thus, D-lactic acid has the potential to be a clinical marker for the diagnosis of these diseases. Hence it is of great significance for clinical diagnosis to assay lactic acids, especially, D-lactic acid level. There is a need for a reliable and selective method to detect and quantify D-lactic acid. This work demonstrates development of a new and highly sensitive analytical method for a simultaneous determination and quantification of L- and D-lactic acid enantiomers. The method demonstrates the application of Supelco’s Astec CHIROBIOTIC® R chiral column in an LC-MS/MS to chirally separate L- and D-lactic acid enantiomers. In addition, the linearity and precision of this method are good. This application is rapid, simple and reproducible for clinical routine detection of L- and D-lactic acid enantiomers.
The L- and D- isomers of lactic acid, both can contribute to metabolic acidosis, but their relative contributions are distinguishing due to their different origins and metabolic pathways. Therefore, it is important to distinguish the isomers of lactic acid in plasma for understanding their relative contributions to metabolic acidosis. However, because of the similar physical and chemical properties of both enantiomers, it is difficult to determine the isomers of lactic acid in biologic samples. Thus, it is necessary to develop a sensitive and reliable method for the separation and quantification of lactic acid enantiomers in urine samples. Currently enzymatic assays withD-lactic acid dehydrogenase are being applied to measure the D-lactic acid in urine. However the enzymatic assays lack sensitivity and specificity in urine controls of newborns, due to low levels of D-lactic acid, that are below the level of quantification by the enzymatic method.
Figure 1. Structure of L- and D- Lactic Acid
The current work has demonstrated a reliable and reproducible analytical method for the simultaneous determination and quantification of L- and D-lactic acid enantiomers in urine by purification and concentration of the organic acids followed by chiral chromatography coupled to tandem MS. This method is rapid, simple and effective for clinical routine detection of L- and D-lactic acid enantiomers. This method deployed the use of Astec CHIROBIOTIC R chiral column, which is available here. With this analytical method, L-lactic acid can be quantified within a range of 2-400 ìm/L and D-lactic acid can be quantified within a range and 0.5-100 µm/L, with accuracy and high precision (Figure 2).
HPLC (Isocratic in an HPLC MS-MS system):
column:Astec CHIROBIOTIC R Chiral Column, 15 cm x 2.1 mm I.D., 5 µm (13019AST)
mobile phase:15% (v/v) 33.3 mM ammonium acetate in H2O and 85% (v/v) acetonitrile
flow rate:0.7 mL/mi
column temp.:4 °C
detector:MS, ESI(-), MRM, m/z 89.1/43.3, 90.1/44.3, 93.1/46.3
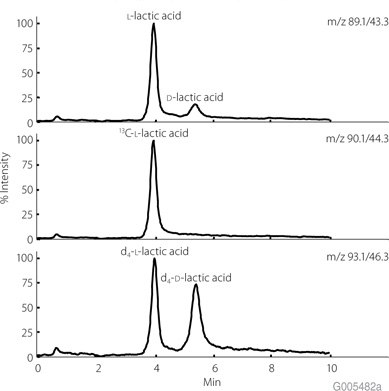
Figure 2.LC MS/MS Chromatogram Showing the Chiral Separation of Urinary L- and D-Lactic Acid using an Astec CHIROBIOTIC® R Chiral HPLC Column HPLC (Isocratic in an HPLC MS-MS system): column: Astec CHIROBIOTIC® R Chiral Column, 15 cm x 2.1 mm I.D., 5 µm (Product No. 13019AST); mobile phase: 15% (v/v) 33.3 mM ammonium acetate in H2O and 85% (v/v) acetonitrile; flow rate: 0.7 mL/min; column temp.: 4 °C; detector: MS, ESI(-), MRM, m/z 89.1/43.3, 90.1/44.3, 93.1/46.3
Conclusion
The Astec CHIROBIOTIC® R chiral column is most suitable for enantio-resolution of hydrophilic acids like alpha-hydroxy/halogenated acids and N-blocked amino acids. This phase’s unique ionic character and H-bond capability play an important role in the chiral recognition mechanism. Also, we offer a variety of other chiral stationary phases that can be used in above capacity to resolve enantiomers of diverse molecules.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?