Traceable Organic Certified Reference Materials for P-31 qNMR
Tools for the Quantification of Phosphorus
Phosphorus plays an important role in physiological processes since it is part of various significant molecules, such as nucleic acids (e.g. DNA), high-energy phosphates (e.g. ATP) and phospholipids. It is essential in the regulation of metabolism, since phosphorylation and dephosphorylation reactions are rapidly occurring processes at the protein level. Studies of physiological pathways, kinetics, metabolomics, and diseases, as well as biomarker discovery, are important fields of investigation, and there is a need for certified reference materials (CRMs) for the quantification of phosphorylated organic compounds and metabolites. The quantification by NMR offers several advantages as it is based on a signal comparison of the analyte with an internal or external reference standard. In contrast to other methods, such as chromatography, the reference standard is independent of the analyte’s chemical structure. Moreover, using very pure primary reference materials, qNMR is a highly accurate method with low measurement uncertainty, which also provides traceability to SI units (Système International d’Unités) and thus offers the possibility of certifying reference materials for 1H or other nuclei, such as 31P.
This article presents the concept of value assignment by 31P qNMR measurements for the development of CRMs and describes different approaches to establish traceability to primary Standard Reference Material from the National Institute of Standards and Technology (NIST SRM) [1]. Water-soluble and phosphorus-containing CRMs are widely available, e.g. NIST SRM 194a (NH4H2PO4), which can be used as a primary standard for establishing traceability in 31P qNMR measurements. Unfortunately, the situation is different for phosphorus-containing primary CRMs that are soluble in common organic solvents, such as dimethylsulfoxide or methanol. No internationally accepted reference standard, such as a primary CRM from a national metrological institute (NMI), is currently available, and therefore, direct traceability to a NIST SRM is not possible in the case of organic candidates. For that reason, the concept was to perform quantification via the protons using 1H qNMR and use the assigned content to determine the purity of an analyte via the phosphorus using 31P qNMR. Phosphonoacetic acid is analyzed as a water-soluble CRM candidate, whereas triphenyl phosphate is a good candidate for use in organic solvents. The substances contain both nuclei, 1H and 31P. For the validation of the concept, triphenyl phosphate and phosphonoacetic acid have been used as 31P qNMR standards to determine the purity of the analyte tris(2-chloroethyl) phosphate.
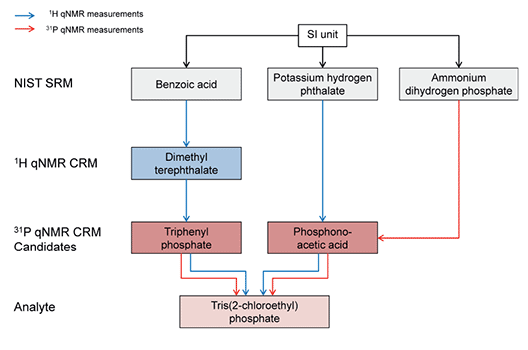
Figure 1.Concept for the quantification of two phosphorus candidate CRMs
Preliminary tests have been performed to check the chemical compatibility between sample and internal standard by acquiring a proton spectrum and, where required, a 31P NMR spectrum of the mixture right after preparation and again after 24 h. It was necessary to exclude potential signal overlaps, as well as reactions between analyte and standard or reactions with the solvent. To ensure that no impurity was lying underneath the peaks of interest, 2D NMR experiments were applied where impurities of less than 0.05% signal intensity portion could be detected. Prior to the quantification measurements, the T1 relaxation times in different deuterated solvents were determined by inversion recovery experiments. Relaxation times should preferably be short, because the relaxation delay is adjusted to at least seven times T1. Since relaxation times may slightly vary in the mixture, the longest value in a mixture is used for calculating the overall relaxation time of the experiment. In a subsequent step, hygroscopy and volatility of the candidate substances were checked, since both attributes have a strong influence on the weighing results, and thus the outcome of the quantification measurement.
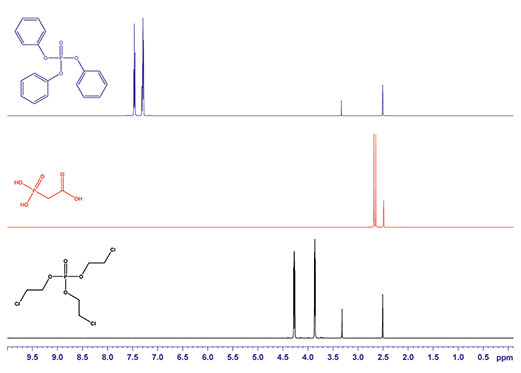
Figure 2.1H NMR spectra for triphenyl phosphate (blue), phosphonoacetic acid (red) and tris(2-chloroethyl) phosphate (black). All substances were dissolved in DMSO-d6.
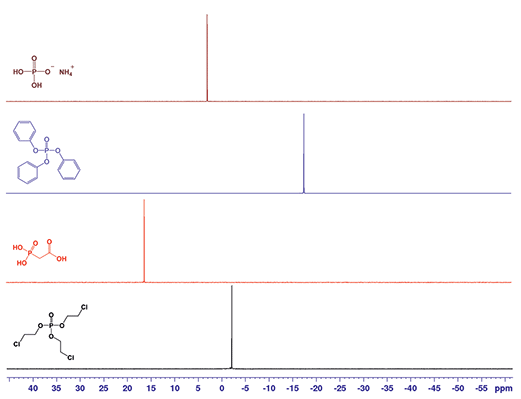
Figure 3.31P NMR spectra for ammonium dihydrogen phosphate in D2O (brown), triphenyl phosphate in CDCl3 (blue), phosphonoacetic acid in D2O (red) and tris(2-chloroethyl) phosphate in CDCl3 (black).
Quantification of the Candidate CRM Phosphonoacetic Acid
To prove the concept that no bias in a qNMR measurement is created when the purity is determined using either proton or phosphorus signals, the quantification of phosphonoacetic acid was performed by 31P and 1H qNMR measurements . In the first experiment , the content of phosphonoacetic acid was determined by 31P qNMR using ammonium dihydrogen phosphate (NIST SRM 194a) as a reference, resulting in a purity value of 99.26% ± 0.75%. The second purity determination was carried out by 1H qNMR measurement using potassium hydrogen phthalate (NIST SRM 84L) as a reference, resulting in a purity value of 99.32% ± 0.17%. These two results deriving from two independent traceability chains and via qNMR measurements of different nuclei are fully consistent and overlap within their expanded measurement uncertainties, as shown in Figure 4.
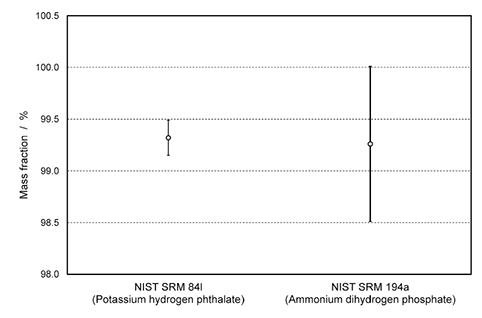
Figure 4.Graphical illustration of purity values and their expanded uncertainties for phosphonoacetic acid: Two different primary references from NIST were used for establishing traceability to SI unit. Potassium hydrogen phthalate was used as internal standard in 1H qNMR measurements and ammonium dihydrogen phosphate in 31P qNMR measurements.
Quantification of the Candidate CRM Triphenyl Phosphate
After the 1H and 31P qNMR experiments for the quantification of phosphonoacetic acid proved the independence of the purity value determined from the nucleus, triphenyl phosphate could be quantified by 1H qNMR. Direct application of benzoic acid as standard reference material in proton NMR was not feasible, due to the overlapping of the signals in the aromatic region. Therefore, the traceability chain was achieved by the use of the CRM dimethyl terephthalate (07038) as an internal reference standard that is traceable to NIST SRM 350b, benzoic acid. The content of triphenyl phosphate was determined to be 99.95% ± 0.22%. Although this value was achieved by 1H qNMR measurements, it can be employed in subsequent 1H and 31P qNMR measurements as proven above. To remove any doubt regarding this conclusion, the concept was proven in a further step, in which a sample containing protons and phosphorus was chosen as the sample for the quantification by the two 31P qNMR CRM candidates.
Quantification of Tris(2-Chloroethyl) Phosphate by 31P qNMR
In the certification concept for phosphorus standards described, the chosen candidate compounds contain both 1H and 31P nuclei. After triphenyl phosphate and phosphonoacetic acid have been quantified via their protons and/or phosphorus, the resulting purity values were used for the subsequent purity determination by 31P qNMR experiments. The quantification of tris(2-chloroethyl) phosphate on the basis of 31P qNMR yielded purities of 98.43% ± 0.66% by using triphenyl phosphate as a reference, and 98.45% ± 0.44% by using phosphonoacetic acid as a reference. Additionally, the quantification of tris(2-chloroethyl) phosphate on the basis of 1H qNMR yielded purities of 98.41% ± 0.53% by using triphenyl phosphate as a reference, and 98.88% ± 0.52% by using phosphonoacetic acid as a reference. All these results derived from different nuclei and different traceability chains are summarized in Figure 5, and the consistency of the data is demonstrated by the overlap of their expanded measurement uncertainties. A potential combination of triphenyl phosphate and phosphonoacetic acid for quantification within the same NMR tube was not possible, due to their differing solubilities (polar and non-polar). It is important to note that the experiments were independent of each other; also, different measurement systems had to be tested in each case to find a suitable reference standard, including selecting the appropriate deuterated solvent and elaborating the appropriate acquisition parameters, such as relaxation times.
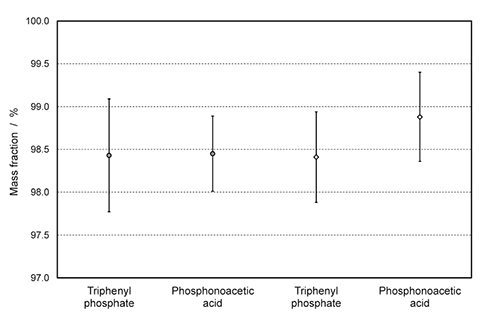
Figure 5.Graphical illustration of purity values and expanded measurement uncertainties for tris(2-chloroethyl) phosphate: Phosphonoacetic acid and triphenyl phosphate are used as internal standards, and the experiments are based on 31P (circle) and 1H (diamond) qNMR measurements.
Measurement Uncertainty
The quantification of the purity in the experiments shown is based on the so-called internal standard method, where the analyte signal is directly compared to an internal reference signal. This approach can be applied not only for 1H, but also for 31P signals. The CRM content is finally expressed as percent mass fraction. It should be noted that even minor uncertainty contributions from air buoyancy correction were taken into account. Therefore, climate data were recorded during each weighing step. The uncertainty calculation is based on well-established guidelines. The combined standard uncertainty is determined by statistical as well as systematic contributions; the statistical contribution arises from the repeatability of weighing and signal integration. Various systematic contributions, such as the air buoyancy correction, balance parameters, molecular masses, and the purity of the reference (expressed as a mass fraction), were taken into account. Phase correction and the integration of the signals were done manually and may differ slightly with the operator. This individual influence is considered in the overall uncertainty budget as “individual integration contribution”. 31P has a relative receptivity of 0.0665, which means it is less sensitive than 1H (receptivity of 1.00), thereby leading to a lower signal-to-noise ratio. This can be compensated for by a higher analyte concentration or a higher number of scans, but it has to be considered that higher analyte concentrations can lead to reduced solubility and therefore higher viscosity or broadening of the signals.
Conclusions
The certification concept for 31P qNMR CRM described has successfully shown how traceability to an SI unit can be established by qNMR using different nuclei. Within this concept, it was proven that purity values of a single material (phosphonoacetic acid (96708)) using 1H or 31P measurements are fully consistent with each other. Taking advantage of this concept, the content of triphenyl phosphate (05498) was determined by 1H qNMR, but the substance with its purity value was subsequently used as 31P qNMR CRM. In an additional experiment, the purity of an exemplary analyte, tris(2-chloroethyl) phosphate, was measured following different traceability chains and solvent systems. Since the results are comparable within the range of their measurement uncertainties, the robustness of the certification concept is demonstrated. A possible application for the usage of these 31P qNMR CRMs is given by the measurements of tris(2-chloroethyl) phosphate, a sample analyte. The work described in this article represents an important step towards a successful method validation for 31P qNMR measurements.
Featured Products
Reference
如要继续阅读,请登录或创建帐户。
暂无帐户?