Western Blotting Sample Preparation
The importance of good sample preparation cannot be stressed enough. By understanding the nature of your starting sample and having a clear picture of the information you wish to derive from your Western blotting experiments, you increase your chances of a successful analysis. The emphasis of this protocol, therefore, is the ground rules of good practice in sample preparation, helping ensure that you get it right from the start.
In principle, all sources of protein, from single cells to whole tissues, as well as extracellular matrices, biological fluids, and proteins secreted in vitro, are open to analysis by Western blotting. Whereas sources such as mammalian cells in suspension are easily disrupted under mild conditions and readily release their proteins, it is more difficult to extract proteins from cells deeply embedded in intact tissues or within solid tumors. Extraction of the proteins from plants, bacteria, and fungi is further complicated by the presence of the rigid, carbohydrate-rich cell wall that surrounds and protects the living cell.
Regardless of the source and protein of interest, however, the aim must be to devise an extraction procedure aggressive enough to access and disrupt the cells without irreversibly altering the very proteins of interest, while at the same time, obtaining a sufficient yield of material at an acceptable level of purity.
Sample Preparation – Be gentle! Stay cool!
- Use extraction procedures that are as mild as possible: Over-vigorous cell or tissue disruption may directly denature the target molecule, form permanent protein complexes, cause chemical modifications, or lead to the release of compartmentalized proteolytic enzymes.
- Extract proteins quickly, on ice if possible, in the presence of a suitable buffer to maintain pH, ionic strength, and stability in order to prevent protein degradation. Pre-chill equipment and keep samples on ice at all times.
Biological matrices are complex. The target protein is likely to be one among many thousands present in the sample, in addition to nucleic acids, polysaccharides, and lipids, all of which may interfere with the analysis. The efforts invested in extraction and purification depend on the end goal; if the aim is to detect a low abundant protein, for example, it may be advisable to affinity isolate that specific protein from the sample using a technique such as immunoprecipitation. On the other hand, the analysis of robust and abundant proteins may be satisfactorily accomplished using virtually native samples.
The choice of extraction method depends primarily on the sample and whether the analysis is targeting all the proteins in a cell or only a component from a particular subcellular fraction.
In addition, as endogenous proteases may be liberated upon cell disruption and may degrade the target molecule, the sample should be protected during cell disruption and subsequent purification by the use of a cocktail of protease inhibitors to avoid uncontrolled protein losses.
Numerous methods are available for disrupting cells and preparing their contents for analysis by Western blotting. Table 1 lists some of the most popular extraction methods and indicates their applicability to the treatment of specific cell or tissue sources. In general, gentle methods are employed when the sample consists of easily lysed cultured cells or blood cells, whereas more vigorous methods are employed for the disruption of more robust bacterial or plant cells, or mammalian cells embedded in connective tissue.
Protein extraction options
Detergent-based Lysis
Detergent lysis is most frequently the method of choice for the treatment of mammalian cells. Cell suspensions are gently centrifuged and resuspended in lysis solution containing detergent. The membranes are solubilized, lysing cells and liberating their contents. Adherent cells such as fibroblasts may be directly solubilized on the tissue culture surface by addition of lysis solution, or alternatively may firstly be scraped from the surface in the presence of a non-lytic buffer using a rubber scalpel, centrifuged, and treated as cell suspensions. The use of a mild, non-ionic detergent such as Triton** X-100, nonyl phenoxypolyethoxylethanol (NP40) or a zwitterionic detergent such as 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), helps ensure that denaturation of target proteins is kept to a minimum.
Freeze/Thaw Lysis
This method is applicable to suspensions of mammalian or bacterial cells. The major attractions of freeze/thaw lysis are simplicity and low cost. Cells are disrupted by the repeated formation of ice crystals and the method is usually combined with enzymatic lysis. The cell suspension may be rapidly frozen using liquid nitrogen. The sample is then thawed, and resuspended by pipetting or gentle vortexing in lysis buffer at room temperature, and the process is repeated several times. Between cycles, the sample is centrifuged, and the supernatant is retained.
Osmotic Shock
This is a very gentle method that may be sufficient for the lysis of suspended mammalian or bacterial cells without the use of a detergent. The method, often combined with mechanical disruption, relies on changing from high to low osmotic medium, and is well- suited to applications in which the lysate is to be subsequently fractionated into subcellular components.
Ultrasonication
This method of protein extraction is most frequently applied to cell suspensions. Cells are disrupted by high-frequency sound waves (typically 20 to 50 kHz) via a probe inserted in the sample. The sound waves generate a region of low pressure, causing disruption of the
membranes of cells in the vicinity of the probe tip. Cell suspensions should be sonicated in short bursts to avoid heating and samples should be cooled on ice between bursts. This is suitable for small-scale sample preparation. Aggregates of proteins (inclusion bodies) must be resolubilized. Although relatively simple, ultrasonication is a stringent method of sample preparation, where generated heat must be continually kept under control and sensitive target molecules may be vulnerable to shearing forces.
Note: For protein preparations, the release of DNA can lead to highly viscous samples that are difficult to process. Viscosity may be reduced by adding DNase.
Mechanical Methods
Proteins may be extracted from cells and tissues using a number of crude but effective "crushing and grinding" measures. For example, cell membranes may be disrupted by liquid shear forces as the sample is forced through a narrow gap: the tighter the gap, the greater the shearing force. This may be achieved manually by dounce homogenization or mechanically by Potter-Elvehjem homogenization. This mild method is excellent for small volumes and cultured cells.
Homogenization of tissues, prepared by chopping or mincing in chilled buffer, may be achieved using a Waring blender or Polytron. The Polytron differs from the Waring blender in that it draws the tissue into a long shaft that contains rotating blades. Different capacity shafts are available, allowing sample sizes as small as 1 mL.
Mortar and pestle: Tissues or cells are normally frozen in liquid nitrogen and ground to a fine powder. The addition of alumina or sand may aid grinding. Cell walls are disrupted by mechanical force.
Glass bead milling: Rapid agitation of cells with fine glass beads disrupts cell walls. Bead milling will lyse most Gram-positive and Gram-negative bacteria, including mycobacteria.
Enzymatic Digestion
Enzymatic methods are frequently used when extracting proteins from bacteria, yeast, or other organisms with cell membranes surrounded by a robust protective structure. The enzymes dissolve cell walls, coats, capsules, capsids, or other structures not easily sheared by mechanical methods alone. Enzymatic digestion is often followed by homogenization, sonication, or vigorous vortexing in a lysis buffer. Enzymatic methods are most commonly used for bacteria and yeast, but may also be used for the extraction of proteins from eukaryotic cells embedded in fibrous tissues, where, for example, collagenase may be appropriate to enhance the breakdown of fibrillar collagen (Table 2).
Explosive Decompression
This technique is most usually applied to bacteria, yeast, plant cells, or other robust samples. Cells are equilibrated with an inert gas, such as nitrogen at high pressure (typically 5500 kPA/800 psi). Using a French press, this method works via a rapid pressure drop when the sample is transferred from a chamber at high pressure through an orifice into a chamber at low pressure. This is a fast and efficient method, suitable for large volumes.
Pre-made Lysis Buffers and Sample Preparation Kits
Cytiva offers a range of products for protein extraction from mammalian cells, yeast, bacteria, and animal tissues.
The Sample Grinding Kit may be used to disrupt small tissue and cell samples for protein extraction. Up to 100 mg of sample per tube may be treated in about 10 min. The kit consists of microcentrifuge tubes, each containing a small quantity of abrasive grinding resin suspended in water, and disposable pestles. The tube is first centrifuged to pellet the resin and water is removed. Then extraction solution of choice and the sample are added to the tube, and the pestle is used to grind the sample. After centrifugation, cellular debris and grinding resin are firmly lodged in the conical bottom of the tube, and the supernatant is easily removed
The illustra™ triplePrep™ Kit is designed for the rapid isolation and purification of high yield genomic DNA, total RNA, and total denatured proteins from undivided samples of animal tissues and mammalian cells. The workflow reduces the overall number of steps, enabling the preparation of all three analytes in less than 1 h. The buffer, columns, and protocol ensure high recovery from limited samples such as biopsies, archived tissues, and tumors.
Mammalian Protein Extraction Buffer is designed for the efficient and gentle extraction of biologically active, total soluble proteins from mammalian cultured cells. This buffer is based on organic buffering agents and can be used for cell suspensions as well as adherent cells.
Yeast Protein Extraction Buffer Kit is useful for the extraction of soluble proteins from yeast cells and is a proprietary improvement on zymolyase-based spheroplast preparation and extraction of soluble proteins from yeast cells. This kit is provided with a protocol to make spheroplasts and remove the lytic enzyme, Zymolyase, prior to lysis and extraction of yeast proteins. The buffer is based on organic buffering agents containing mild non-ionic detergents, and a proprietary combination of various salts and agents to enhance extraction and stability of 28-9998-97 AB 19 proteins. A ready-to-use Zymolyase preparation is also provided. Depending on the application, additional agents such as reducing agents, chelating agents, and protease inhibitors may be added. The Yeast Protein Extraction Buffer Kit eliminates the need for laborious glass bead lysis of yeast cells.
The 2-D Fractionation Kit simplifies analysis of complex protein mixtures by reducing the amount and number of protein species loaded into the gel matrix. Fractionation makes it possible to isolate groups of proteins, or fractions from the total proteome. This allows for improved resolution when an individual fraction is analyzed, provides less crowded 2-D maps, simplifies analysis and interpretation, and increases the chances of discovering novel proteins of diagnostic or therapeutic interest. These scalable kits help ensure high sample recovery and are compatible with downstream separation techniques, such as 2-D gel electrophoresis.
Protecting Your Samples
Protease inhibitors must be included in lysis buffers to prevent degradation of proteins following the release of endogenous proteases during the process of cell lysis.
Protease Inhibitor Mix: Sample preparation often requires the inhibition of protease activity. Cytiva offers this unique combination of competitive and non-competitive protease inhibitors, which protect proteins from proteolysis during purification from animal tissues, plant tissues, yeast, and bacteria. The cocktail, containing inhibitors of serine, cysteine, and calpain proteases, effectively inhibits over 95% of the protease activity and has been specifically developed for sample preparation in 2-D gel electrophoresis studies. Optionally, ethylenediaminetetraacetic acid (EDTA) may be added to inhibit metalloproteases.
While it is important to maintain proteases in an inactive state during protein extraction (Table 3), other potentially compromising contaminants should also be considered. For example, if the objective of your Western blot is to detect phosphorylated proteins, it is important to protect your sample from the dephosphorylating action of phosphatases liberated into the lysate during sample preparation. One way to protect your sample is by adding a phosphatase inhibitor, such as sodium vanadate to your lysis buffer. It may also be necessary to protect your proteins against unwanted modifications, such as acetylation, ubiquitinylation, or glycosylation.
Sample cleanup
It is not usually necessary to treat samples prior to 1-D gel electrophoresis, but it is very important in 2-D gel electrophoresis applications. However, if you experience problems with separation, such as blurred bands, sample cleanup may improve performance by removing potentially interfering compounds such as nucleic acids, polysaccharides, and salts. The addition of DNase, for example, may be used to counter problems with viscosity caused by the release of nucleic acids. Table 4 provides a list of common contaminants and options for dealing with them.
Sample Cleanup Products
SDS-PAGE Clean-Up Kit is designed for the preparation of samples that are difficult to analyze due to the presence of salts or a low protein concentration (Figure 1). This kit uses a combination of a precipitant and co-precipitant to quantitatively precipitate the sample proteins while leaving interfering substances such as detergents, salts, lipids, phenolics, and nucleic acids in solution. Proteins are pelleted by centrifugation. The pellet is washed further to remove non-protein contaminants and centrifuged again. The resultant pellet is resuspended, mixed with SDSPAGE sample buffer, and heated. The sample is then ready for SDS-PAGE. The procedure can be completed in under 2 h.

Figure 1. Comparison of SDS-PAGE Clean-Up Kit with ethanol precipitation. (A) Urinary protein precipitated with 10 volumes of ethanol. (B) Urinary protein precipitated with SDS-PAGE Clean-Up Kit. Gel: 8 × 9 cm, 12.5% acrylamide, 0.1% SDS, run on SE 260 Mini-Vertical Unit. Stain: Coomassie** Blue R-250.
2-D Clean-Up Kit is designed to prepare samples for 2-D gel electrophoresis (Figure 2), but can also be used in Western Blotting applications. The reagents quantitatively precipitate proteins while leaving interfering substances, such as detergents, salts, lipids, phenolics, and nucleic acids, in solution. Treatment of the sample with 2-D Clean-Up Kit greatly improves the quality of 2-D gel electrophoresis results, reducing streaking, background staining, and other artefacts. For more information on 2-D Clean-Up Kit, see the 2-D Electrophoresis, Principles and Methods Handbook from Cytiva (2).
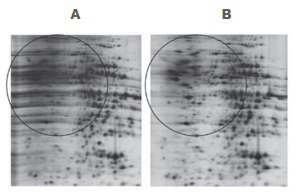
Figure 2.2-D Clean-Up Kit eliminates most of the horizontal streaking caused by residual SDS. Sample: Rat liver extracted with 4% SDS, 40 mM Tris base. First dimension: Approximately 20 μg of rat liver protein, Immobiline™ DryStrip (pH 4-7, 7 cm). Ettan™ IPGphor™ 3 Isoelectric Focusing Unit unit 17.5 kVh. Second dimension: SDS-PAGE (12.5%), run on SE 260 Mini-Vertical Unit (8 × 9 cm gel). Stain: PlusOne™ Silver Staining Kit, Protein.
Depletion of High Abundance Protein from Serum or Plasma Samples
When investigating plasma or serum by Western blotting, abundant plasma proteins, such as albumin and IgG can obscure the signals of less abundant proteins. Prepacked columns, such as HiTrap™ Albumin & IgG Depletion are designed to deplete samples of these potentially problematic proteins, removing >95% albumin and >90% IgG, respectively.
HiTrap Albumin & IgG Depletion 1 mL column is designed for the depletion of albumin and IgG from sample volumes of approximately 150 μL of undiluted human plasma or serum, containing normal levels of albumin (~40 mg /mL) and IgG (~15 mg/mL). The depletion procedure takes approximately 35 min, and can be performed using a liquid chromatography system from the ÄKTA™ design platform, a peristaltic pump, or manually with a syringe. When working with smaller volumes, Albumin & IgG Depletion SpinTrap™, designed for volumes of ~50 μL of human plasma or serum, is recommended.
Desalting and Concentrating Samples
Before applying your sample to an electrophoresis gel, it is important that the solvent does not contain an excessive concentration of salts or other low molecular weight contaminants. High salt levels in samples may cause the proteins to migrate in inconsistent and unpredictable patterns. Desalting may be achieved in a single step based on gel filtration, and at the same time transferring the sample into the desired buffer. However, desalting and buffer exchange procedures often result in sample dilution. In electrophoresis applications, a relatively high sample concentration is needed for good results and sample concentration may thus be necessary. A sample can be concentrated efficiently and easily by membrane ultrafiltration.
Some desalting and concentrating products provided by Cytiva are summarized in Table 5.
Determination of total protein concentration
When comparing the amount of protein from samples run in different lanes within the same gel or between gels, it is very important that all the lanes have been loaded with the same total amount of protein. A two-fold increase in the expression level of a specific protein in one lane will be completely masked if a comparative lane contains twice the amount of total protein (or will even appear to be reduced in expression if the comparitor lane contains more than twice the amount).
Several spectrophotometric methods are routinely used to determine the concentration of protein in a solution (3). These include measurement of the intrinsic ultraviolet (UV) absorbance of the protein as well as methods based on a protein-dependent color change, such as the classic, copper-based Lowry assay (4), the Smith copper/bicinchoninic assay (BCA) (5) and the Bradford dye assay (6). Although widely used, none of the these procedures are particularly convenient.
UV absorbance, for example, requires access to a pure protein of a known extinction coefficient, in a solution free of interfering (UV absorbing) substances. The approximate concentration of a protein in solution (assuming the use of a cuvette with a path length of 1 cm) can be estimated by using either of the following equations;
A280 = 1 A1 (mL/cm mg) × [Conc.] (mg/mL) × 1 (cm)
A205 = 31 A1 (mL/cm mg) × [Conc.] (mg/mL) × 1 (cm)
1 A280 represents light absorbed by proteins at 280 nm, primarily a result of the presence of ringed amino acids tyrosine and tryptophan. A205 represents light absorbed by proteins at 205 nm, primarily the result of peptide bonds between amino acids.
Different proteins, however, have widely different extinction coefficients at both 280 and 205 nm, and concentration estimates obtained in this way are at best a rough estimate. UV absorbance requires that the protein solution is free of other UV-absorbing substances, such as nucleic acids, and that the measurements are carried out using a quartz cuvette.
Copper/BCA assays are based on reduction of Cu2+ to Cu+ by amides. Although quite accurate, these assays require freshly prepared reagent solutions, which must be carefully measured and mixed during the assay. This is followed by lengthy, precisely timed incubations at closely controlled, elevated temperatures, and then immediate absorbance measurements. Both assays may be affected by other substances frequently present in biochemical solutions, including detergents, lipids, buffers, and reducing agents (3). This requires that the assays also include a series of standard solutions, each with a different, known concentration of protein, but otherwise having the same composition as the sample solutions.
The Bradford dye assay is based on the equilibrium between three forms of Coomassie Blue G dye. Under strongly acidic conditions, the dye is most stable in its double protonated form (red). Upon binding to protein, however, it is most stable in an unprotonated form (blue).
In comparison with the other assays described above, the Bradford dye assay is faster, involves fewer mixing steps, does not require heating, and gives a more stable colorimetric response. The assay is prone, however, to influence from non-protein sources, particularly detergents, and becomes progressively less linear at the high end of its useful protein concentration range. The response also varies with the structure of the protein. These limitations make it necessary to use protein standard solutions in this assay.
The Bradford dye reagent reacts primarily with arginine residues and, to a lesser extent, with histidine, lysine, tyrosine, tryptophan, and phenylalanine residues. The assay is thus less accurate for basic or acidic proteins and is more sensitive to bovine serum albumin than "average" proteins, by about a factor of two. IgG is the preferred protein standard for the Bradford dye assay.
Products for Determination of Total Protein Concentration
The use of UV/visible spectrophotometers is widespread in protein analysis. The Ultrospec™ spectrophotometer series from Cytiva, for example, provides modules for protein determination, enzyme activity kinetics, DNA and RNA quantitation and fraction analysis. These units are compatible with the traditional protein determination methods described above. The instruments are equipped with an eight-position sample changer and are optimal for protein concentration determination with the 2-D Quant Kit.
In addition, the Novaspec™ Plus visible spectrophotometer has stored methods for protein concentration determination by Bradford dye assay, BCA, Biuret, and Lowry assays, as well as basic modes of absorbance, transmittance, OD600, and concentration.
2-D Quant Kit, despite its name, can be used in many different applications including the accurate determination of protein concentration in samples. The procedure works by quantitatively precipitating proteins while leaving interfering substances behind. The assay is based on the specific binding of cupric ions to the polypeptide backbone of any protein present. Precipitated proteins are resuspended in a copper-containing solution and unbound copper is measured with a colorimetric agent. The absorbance at 480 nm is inversely related to the protein concentration. The assay has a linear response to protein concentrations in the range of 0 to 50 μg/mL, using a recommended sample volume of 1 to 50 μL. In addition, 2-D Quant Kit is compatible with most reagents employed in the many techniques described for sample preparation, such as SDS.

**Third party trademark
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?