Purification Using GST HiTrap® 1 mL and 5 mL Columns: GSTrap™ 4B, GSTrap™ FF, and GSTrap™ HP
GSTrap affinity columns are specially designed 1 mL and 5 mL HiTrap columns packed with Glutathione Sepharose High Performance, Glutathione Sepharose 4 Fast Flow, or Glutathione Sepharose 4B media. Refer to the selection guide in Table 3.1 for purification options using these columns and to Characteristics of Glutathione Sepharose and HiTrap Benzamidine FF (high sub) media and columns for a summary of their characteristics.
Sample application, washing, and elution can be performed using a syringe with a supplied connector, a peristaltic pump, or a liquid chromatography system such as ÄKTA. For easy scale-up, two to three columns can be connected to each other in series simply by screwing the end of one column into the top of the next. Figure 1 shows a schematic representation of the simple steps needed for successful purification using GSTrap columns.
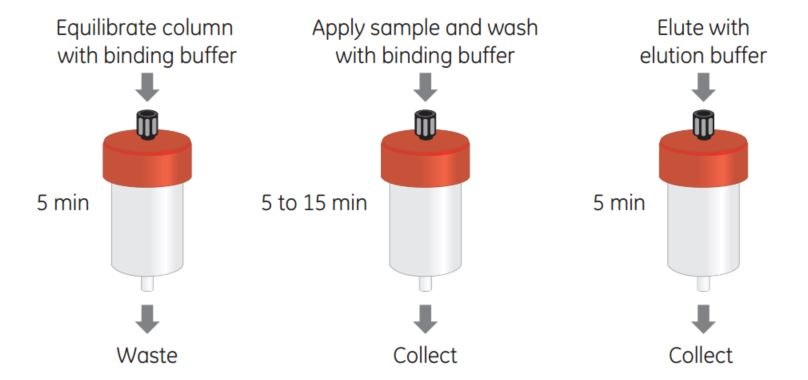
Figure 1.Simple purification of GST-tagged proteins using a GSTrap column.
GSTrap columns are made of polypropylene, which is biocompatible and noninteractive with biomolecules. The top and bottom frits are manufactured from porous polyethylene. The columns are delivered with a stopper on the inlet and a snap-off end on the outlet. Each package includes all necessary components for connection of the columns to different types of equipment. Note that GSTrap columns cannot be opened or refilled.
GSTrap columns are directly compatible with existing purification protocols for GST-tagged proteins, including on-column proteolytic cleavage methods. If removal of the GST moiety is required, the tagged protein can be digested with an appropriate site-specific protease while it is bound to the medium or, alternatively, after the elution.
One of the GST media, Glutathione Sepharose 4 Fast Flow, is also available in prepacked 20 mL GSTPrep FF 16/10 columns. All three GST media are available in bulk packs (varying from 10 to 500 mL) for packing in an empty column of the user’s choice.
The chromatography media are very stable and the purification process very reproducible. This can be seen from the results of an experiment in which E. coli homogenates containing GST-hippocalcin (Mr 43 000) were repeatedly purified 10 times on the same column without cleaning between runs (Figure 2). The 10 overlaid chromatograms show a near perfect match, with little or no variation in binding capacity and indicating stability of the medium. SDS-PAGE analysis (Figure 2) confirmed no changes in purity or binding capacity after these 10 runs.
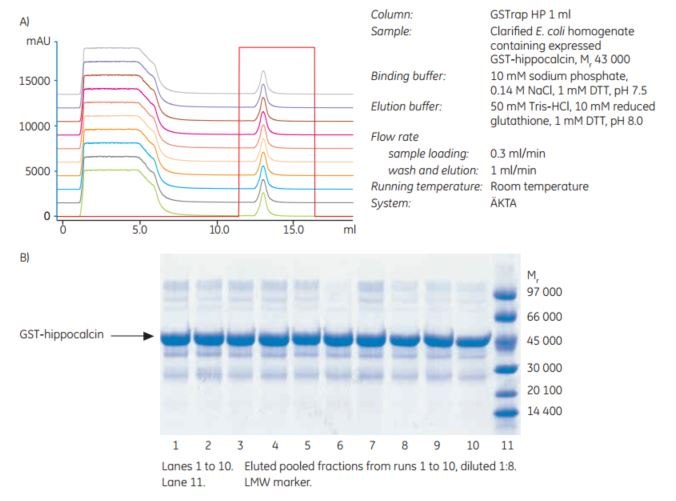
Figure 2.(A) Confirmation of the stability of Glutathione Sepharose High Performance prepacked in 1 mL GSTrap HP columns. Chromatographic overlay of 10 repetitive purifications. (B) Coomassie-stained nonreduced SDS-polyacrylamide gel (ExcelGel SDS Gradient 8–18%) of pooled fractions from repetitive purification runs shown in (A).
Before starting the procedure, refer to general considerations for purification of GST-tagged proteins.
Procedure
- Fill the syringe or pump tubing with binding buffer. Remove the stopper and connect the column to the syringe (use the connector supplied), laboratory pump, or chromatographic system “drop to drop” to avoid introducing air into the column.
- Remove the snap-off end at the column outlet.
- Equilibrate the column with 5 column volumes of binding buffer.
- Apply the pretreated sample using a syringe fitted to the Luer connector or by pumping it onto the column. For best results, use a flow rate of 0.2 to 1 mL/min (1 mL column) and 0.5 to 5 mL/min (5 mL column) during sample application1.
- Wash with 5 to 10 column volumes of binding buffer or until no material appears in the effluent. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for washing.
Optional: Collect the flowthrough (in 1 mL fractions for the 1 mL column and 2 mL fractions for the 5 mL column) and reserve until the procedure has been successfully completed. Retain a sample for analysis by SDS-PAGE or by CDNB assay to check for any loss of unbound target protein.
- Elute with 5 to 10 column volumes of elution buffer. Maintain a flow rate of 0.2 to 1 mL/min (1 mL column) and 0.5 to 5 mL/min (5 mL column) for elution.
- After elution, regenerate the column by washing it with 3 to 5 column volumes of binding buffer. The column is now ready for a new purification.
1 One mL/min corresponds to approximately 30 drops/min when using a syringe with a HiTrap 1 mL column, and 5 mL/min corresponds to approximately 120 drops/min when using a HiTrap 5 mL column.
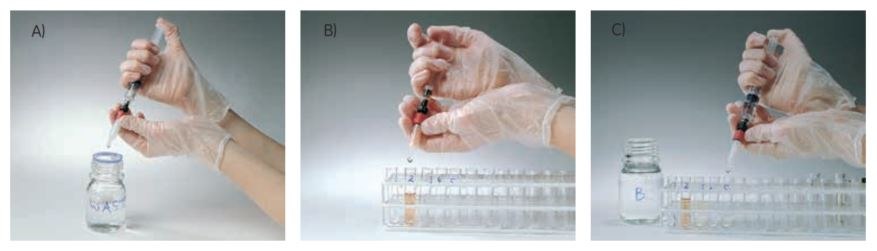
Figure 3.Using a GSTrap column with a syringe. A) Prepare buffers and sample. Remove the column’s top cap and snap off the end. B) Load the sample and begin collecting fractions. C) Wash and elute, continuing to collect fractions.
Volumes and times used for elution may vary among tagged proteins. Additional elutions with higher concentrations of glutathione may be required. Flowthrough, wash, and eluted material from the column should be monitored for GST-tagged proteins using SDS-PAGE in combination with Western blotting, if necessary.
Flow rate will affect the binding and elution of GST-tagged proteins to the chromatography medium. Due to the relatively slow binding kinetics between GST and glutathione, it is important to keep the flow rate low for maximum binding capacity. Protein characteristics, pH, and temperature are other factors that may affect the binding capacity. However, when working with sensitive proteins, higher flow rates are recommended to minimize purification time. Combining two or three columns in tandem would increase residence time for sample passing the column, thus allowing higher flow rates to be used.
The reuse of GSTrap HP, FF, or 4B columns depends on the nature of the sample and should only be performed with identical samples to prevent cross-contamination.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?