Gel Filtration Markers Kit for Protein Molecular Weights 12,000-200,000 Da
MWGF200
Storage Temperature –20 °C
Product Description
Gel filtration chromatography is an established method for determining the size and molecular mass of proteins. Fractionation is based on the diffusion of molecules into the pores of the resin. Larger proteins do not enter the pores of the resin as readily, but pass through the fluid volume of the column faster than smaller proteins. These protein molecules elute from the column in order of decreasing molecular mass.
The molecular mass determination of unknown proteins is made by comparing the ratio of Ve/Vo for the protein in question to the Ve/Vo of protein standards of known molecular mass (Ve is the elution volume and Vo is the void volume). The Vo of a given column is based on the volume of effluent required for the elution of a large molecule such as blue dextran (molecular mass of ~2,000 kDa, Catalog No. D4772). Plotting the logarithms of the known molecular masses of protein standards versus their respective Ve/Vo values produces a linear calibration curve.
Ve/Vo is essentially independent of column size and protein concentration, but may be temperature dependent for some proteins. Unreliable molecular masses may be obtained if the protein forms a complex with the gel, contains a large amount of carbohydrate, aggregates to larger complexes, or dissociates into subunits under the conditions used.1 The molecular mass of an impure protein may be determined using this procedure if a specific detection test is available for the protein.
The procedure for determining molecular masses using gel filtration chromatography as outlined in this bulletin is a modification of published methods.1,2 The protein standards in this kit may be suitable for use in other chromatographic systems including HPLC, although some buffer systems seem to alter the elution volumes of albumin (A8531) and carbonic anhydrase (C7025). The proteins in the MWGF200 Kit have a range of molecular masses from 12.4–200 kDa.
Precautions and Disclaimer
This product is for R&D use only, not for drug, household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.
Storage/Stability
Store the kit at –20 °C.
Procedure
Use of MWGF200 For Gel Filtration Chromatography
- Buffer and Resin – It is recommended to use 50 mM Tris-HCl, pH 7.5,with 100 mM KCl as the equilibration buffer with a 90 cm x 1.6 cm Sephacryl® S-200-HR (Catalog No. S200HR) column at 2–8 °C. For information on resin preparation, column packing, and equilibration contact Technical Service.
- Void Volume (Vo) Determination – Dissolve the blue dextran in equilibration buffer containing 5% glycerol at a concentration of 2 mg/ml. This concentration of blue dextran will give an A280 of ~1.0 in the peak fraction. Glycerol is added to increase the density of the solution, but its use is optional.
The recommended sample volume is less than 2% of the total gel bed volume. Carefully apply the blue dextran sample to the column (avoid disturbing the gel bed surface) to determine Vo and to check column packing. Immediately after applying the sample, begin collecting fractions of 0.5–1.5% of the total gel bed volume. The flow rate should be ~7% of the column volume per hour. Skewing of the blue dextran band represents a fault in the column, although some tailing is normal. The leading peak indicates the void volume. Determine spectrophotometrically the elution volume for blue dextran (Vo for the column) at 280 nm or 610 nm by measuring the volume of effluent collected from the point of sample application to the center of the effluent peak.
Notes: Mixing blue dextran with kit standards or sample proteins is not recommended since many proteins bind to blue dextran.
Prepare protein standards and blue dextran fresh.3 Occasionally some aggregated protein may appear at the void volume.
- Elution Volume (Ve) Determination for Protein Standards – Dissolve individual protein standards in equilibration buffer containing 5% glycerol (see Table 1). If, upon reconstitution, any of the protein solutions contain insoluble material then filter the protein solution through a 0.45 or 0.2 mm filter. The loss of protein from this filtration is negligible. For a 90 cm x 1.6 cm column, the application of 2.0 ml of individual samples at the recommended concentration (Table 2) gives an A280 of ~1 in the peak fraction.
The following proteins may be mixed and run together on the columns:
Cytochrome c and β-amylase
Carbonic anhydrase and alcohol dehydrogenase
Apply protein standards to the column using the same sample volume and flow rate as used for the blue dextran sample. The elution of the standard proteins may be followed by absorbance readings at 280 nm. Determine the Ve for the protein standards by measuring the volume of effluent collected from the point of sample application to the center of the effluent peak.
- Standard Curve – Plot molecular mass vs. Ve/Vo for each respective protein standard on semilog paper (Figure 1).
- Elution Volume (Ve) Determination for an Unknown Protein – Apply the unknown sample to the column at an appropriate concentration using the same sample volume, fraction size, and flow rate as used for the blue dextran and the protein standards. Determine the Ve of the unknown using the same methods applied to the standards. Calculate the Ve/Vo for the unknown and determine its molecular mass from the standard curve.
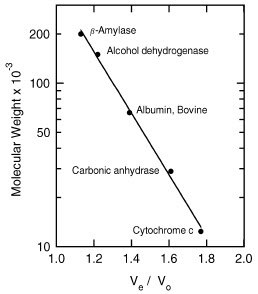
Figure 1.Typical calibration curve obtained with proteins from the MWGF200 Kit run on Sephacryl S-200-HR.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?