Plant RNA/DNA Purification Kit
Product No. E4788

Norgen’s Plant RNA/DNA Purification Kit provides a rapid method for the isolation and purification of total RNA and DNA simultaneously from a single sample of plants. The total RNA and DNA (including genomic DNA) and are both column purified in under 30 minutes using a single column. It is often necessary to isolate total RNA and genomic DNA from a single plant sample, such as for studies of gene expression, mutant or transgenic plant characterization, and host plant-pathogen characterization. Traditionally the RNA and DNA would be isolated from different aliquots of the same sample, however this novel technology will allow for their simultaneous isolation from the same sample. This will not only save time, but will also be of a great benefit when isolating RNA and DNA from precious, difficult to obtain or very small samples. Furthermore, gene expression analysis will be more reliable since the RNA and DNA are derived from the same sample, therefore eliminating inconsistent results.
Norgen’s Purification Technology
Purification is based on spin column chromatography using Norgen’s proprietary resin as the separation matrix. The process involves first lysing the cells or tissue of interest with the provided Lysis Solution (please see the flow chart). The Lysis Solution contains detergents, as well as large amounts of a chaotropic denaturant that will rapidly inactivate any RNases that are present. A heat treatment is performed to ensure complete lysis. Ethanol is then added to the lysate, and the solution is loaded onto a spin-column. Norgen’s resin binds nucleic acids in a manner that depends on ionic concentrations, thus only the RNA and DNA will bind to the column while the proteins are removed in the flowthrough. Next, an optional step can be carried out in which the genomic DNA can be digested allowing for a more pure RNA sample to be isolated. The bound nucleic acid is then washed three times with the provided Nucleic Acid Wash Solution in order to remove any impurities, and the purified RNA and/or DNA is eluted with the Nucleic Acid Elution Buffer.
The kit purifies all sizes of RNA, from large mRNA and ribosomal RNA down to microRNA (miRNA) and small interfering RNA (siRNA). The purified RNA is of the highest integrity, and can be used in a number of downstream applications including real time PCR, reverse transcription PCR, Northern blotting, RNase protection and primer extension, and expression array assays. The genomic DNA is of the highest quality, and can be used in PCR reactions, sequencing, Southern blotting and SNP analysis.
Specifications
* average yields will vary depending upon a number of factors including species, growth conditions used and developmental stage.
Kit Components
Advantages
- Fast and easy processing using rapid spin-column format
- All columns for total RNA and genomic DNA purification provided
- Isolate total RNA, from large rRNA down to microRNA (miRNA)
- No phenol or chloroform extractions
- Isolate high quality genomic DNA and total RNA
Storage Conditions and Product Stability
All solutions should be kept tightly sealed and stored at room temperature. These reagents should remain stable for at least 1 year in their unopened containers.
Precautions and Disclaimers
This kit is designed for research purposes only. It is not intended for human or diagnostic use.
Ensure that a suitable lab coat, disposable gloves and protective goggles are worn when working with chemicals. For more information, please consult the appropriate Material Safety Data Sheets (MSDSs). These are available as convenient PDF files online at www.norgenbiotek.com.
Customer-Supplied Reagents and Equipment
You must have the following in order to use the Plant RNA/DNA Purification Kit:
- Benchtop microcentrifuge
- 96 - 100% ethanol
- 70% ethanol
- Cell disruption tools such as mortar and pestle, rotor-stator homogenizer or bead mills
- Water bath or incubator heated to 65 °C
- β-mercaptoethanol (Optional)
- RNase-free DNase I (Optional)
- RNase A (Optional)
- Liquid nitrogen (Optional)
Working with RNA
RNases are very stable and robust enzymes that degrade RNA. Autoclaving solutions and glassware is not always sufficient to actively remove these enzymes. The first step when preparing to work with RNA is to create an RNase-free environment. The following precautions are recommended as your best defense against these enzymes.
- The RNA area should be located away from microbiological work stations
- Clean, disposable gloves should be worn at all times when handling reagents, samples, pipettes, disposable tubes, etc. It is recommended that gloves are changed frequently to avoid contamination
- There should be designated solutions, tips, tubes, lab coats, pipettes, etc. for RNA only
- All RNA solutions should be prepared using at least 0.05% DEPC-treated autoclaved water or molecular biology grade nuclease-free water
- Clean all surfaces with commercially available RNase decontamination solutions, such as RNase AWAY® (83931)
- When working with purified RNA samples, ensure that they remain on ice during downstream applications
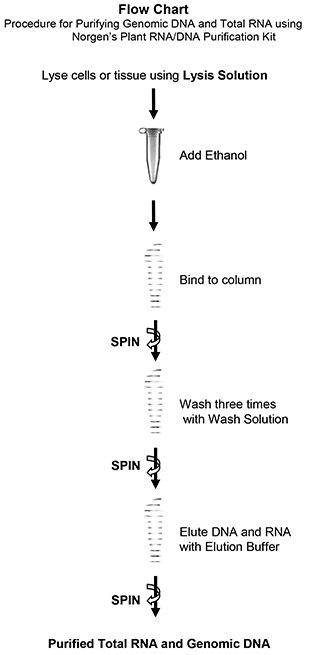
Procedures
All centrifugation steps are carried out in a benchtop microcentrifuge. Various speeds are required for different steps, so please check your microcentrifuge specifications to ensure that it is capable of the proper speeds. All centrifugation steps are performed at room temperature. The correct rpm can be calculated using the formula:

where RCF = required gravitational acceleration (relative centrifugal force in units of g); r = radius of the rotor in cm; and RPM = the number of revolutions per minute required to achieve the necessary g-force.
Notes Prior to Use
- All centrifugation steps are carried out in a benchtop microcentrifuge at 14,000 x g (~ 14,000 RPM) except where noted. All centrifugation steps are performed at room temperature.
- A variable speed centrifuge should be used for maximum kit performance. If a variable speed centrifuge is not available a fixed speed centrifuge can be used, however reduced yields may be observed.
- Ensure that all solutions are at room temperature prior to use.
- Prepare a working concentration of the Nucleic Acid Wash Solution by adding 90 mL of 95 - 100 % ethanol (provided by the user) to the supplied bottle containing the concentrated Nucleic Acid Wash Solution. This will give a final volume of 120 mL. The label on the bottle has a box that may be checked to indicate that the ethanol has been added.
- (Optional): For larger plant samples, or samples with high starch or polysaccharide content, we recommend the use of β-mercaptoethanol during lysis. Add 10 µL of β-mercaptoethanol (provided by the user) to each 1 mL of Lysis Solution required. β-mercaptoethanol is toxic and should be dispensed in a fume hood.
- Pre-heat a water bath or an incubator to 65 °C
- The optimal input of plant tissue is 50 mg or 5 x 106 plant cells. However, for most species, up to 100 mg of tissue may be processed.
- Both fresh and frozen plant samples can be used for this protocol. Samples should be flash-frozen in liquid nitrogen and transferred immediately to a -70 °C freezer for longterm storage. Do not allow frozen tissues to thaw prior to grinding with the mortar and pestle in order to ensure that the integrity of the RNA is not compromised.
- It is important to work quickly when purifying RNA.
- Lysate Preparation
- Transfer ≤50 mg of plant tissue or 5 x 106 plant cells into a mortar that contains 600 µL of Lysis Solution. Grind the sample until the tissue is completely macerated. Alternatively, other homogenization methods can be used with this procedure, including grinding with liquid nitrogen or a bead system. If an alternative method is used, add 600 µL of Lysis Solution to the sample immediately after homogenization and vortex for 20 seconds to mix.
- Using a pipette, transfer the lysate into an RNase-free microcentrifuge tube (provided by user).
- Incubate the lysate at 65 °C for 10 minutes. Mix occasionally by inverting the tube a few times.
- Spin the lysate for 2 minutes to pellet any cell debris. Transfer the supernatant to another RNase-free microcentrifuge tube. Note the volume of the supernatant/lysate.
Note: Ensure the supernatant is free of cell debris. Depending on the plant species Step 1.4 may need to be repeated in order to obtain clean supernatant. - Add an equal volume of 70% ethanol (provided by the user, 100 µL of ethanol is added to every 100 µL of lysate). Vortex to mix. Proceed to Step 2.
- Binding Nucleic Acids to Column
- Assemble a column with one of the provided collection tubes.
- Apply up to 600 µL of the clarified lysate with ethanol onto the column and centrifuge for 1 minute. Discard the flowthrough and reassemble the spin column with the collection tube.
Note: Ensure the entire lysate volume has passed through into the collection tube by inspecting the column. If the entire lysate volume has not passed, spin for an additional minute. - Depending on your lysate volume, repeat step 2.2 if necessary.
- DNase Treatment (Optional)
This optional step is carried out if genomic DNA-free RNA is required. It is recommended that Norgen’s RNase-Free DNase I Kit (Product No. 25710) be used for this step.- Apply 400 µL of Nucleic Acid Wash Solution to the column and centrifuge for 2 minutes. Discard the flowthrough.
Note: Ensure the entire wash solution has passed through into the collection tube by inspecting the column. If the entire wash volume has not passed, spin for an additional minute. - Apply 100 µL of Enzyme Incubation Buffer containing 15 µL of Norgen’s RNase-Free DNase I (Product No. 25710) to the column and centrifuge for 1 minute at 14,000 x g (~14,000 RPM). If using an alternative DNAse I, apply 100 µL of Enzyme Incubation Buffer containing 25 units of DNase I to the column and centrifuge for 1 minute.
Note: Ensure that the entire DNase I solution passes through the column. Repeat the step if needed. At this point, genomic DNA can be isolated instead of the total RNA. If you wish to isolate RNA-free genomic DNA, apply 100 µL of Enzyme Incubation Buffer containing 10 units of RNase A (user provided) to the column and proceed as written below. - After the centrifugation in Step 2, pipette the flowthrough that is present in the collection tube back onto the top of the column.
Note: Ensure Step 3.3 is performed in order to ensure maximum DNAse activity and to obtain maximum yields of RNA. - Incubate the whole unit at room temperature for 15 minutes.
- Proceed to Step 4.3 (2nd Column Wash) without further centrifugation.
- Apply 400 µL of Nucleic Acid Wash Solution to the column and centrifuge for 2 minutes. Discard the flowthrough.
- Column Wash
- Apply 500 µL of Nucleic Acid Wash Solution to the column and centrifuge for 1 minute.
Note: Ensure the entire wash solution has passed through into the collection tube by inspecting the column. If the entire wash volume has not passed, spin for an additional minute. - Discard the flowthrough and reassemble the column with the collection tube.
- Repeat steps 4.1 and 4.2 to wash column a second time.
- Wash column a third time by adding another 500 µL of Wash Solution and centrifuging for 1 minute.
- Discard the flowthrough and reassemble the spin column with its collection tube.
- Spin the column for 2 minutes in order to thoroughly dry the resin. Discard the collection tube.
- Apply 500 µL of Nucleic Acid Wash Solution to the column and centrifuge for 1 minute.
- Nucleic Acid Elution
- Place the column into a fresh 1.7 mL Elution tube provided with the kit.
- Add 150 µL of Nucleic Acid Elution Buffer to the column.
Note: If only RNA is being isolated, reduce the volume of Nucleic Acid Elution Buffer to 100 µL. - Centrifuge for 2 minutes at 200 x g (~2,000 RPM), followed by a 1 minute spin at 14,000 x g (~14,000 RPM). Note the volume eluted from the column. If the entire volume has not been eluted, spin the column at 14,000 x g (~14,000 RPM) for 1 additional minute.
Note: For maximum nucleic acid recovery, it is recommended that a second elution be performed into a separate microcentrifuge tube (Repeat Steps 5.2 and 5.3).
- Storage of DNA and RNA
The purified nucleic acids may be stored at –20 °C for a few days. It is recommended that samples be placed at –70 °C for long term storage.
Troubleshooting Guide
Related Products
如要继续阅读,请登录或创建帐户。
暂无帐户?