Analysis of Alkylphenols Using New Internal Standards
Sigma-Aldrich offers these useful isotopically labeled compounds for GC-MS and LC-MS applications
Alkylphenols are starting materials for the synthesis of alkylphenol ethoxylates, which are widely used as non ionic tensides, dispersive agents in paper and leather manufacturing, emulsifiers for pesticide formulations and as auxiliary agents for drilling and flotation. The most important members are ethoxylates of nonylphenol (NP) and octylphenol (OP). The number of ethoxylate units can be as high as 100. Alkylphenol ethoxylates are produced in huge quantities; the annual worldwide usage is estimated to be around 600,000 tons1.
As a consequence of the wide-spread use for more than forty years, alkylphenols and alkylphenol ethoxylates have become ubiquitous environmental contaminants2, 3 and have even been found in foodstuffs4. Under environmental conditions, the long-chain alkylphenol ethoxylates degrade quickly to the corresponding alkylphenols. The physical-chemical properties of alkylphenols are the reason for the special relevance to aquatic habitats. First, they are highly toxic to many aquatic organisms. Second, compared to alkylphenol ethoxylates OP and NP are highly persistent and show a high potential for bioaccumulation because of their low water solubility. The environmental consequences of this bioaccumulation are of serious concern: Alkylphenols exhibit estrogen-like activity and disrupt male fertility in fish and aquatic mammals5. The same mechanism operates for short-chain ethoxylates of OP and NP with one to two ethoxy units.
The environmental impact of OP and NP and their mono- and diethoxylates have prompted their inclusion in national and international legislation with corresponding monitoring programs. One of the most important agreements is the OSPAR Convention for the Protection of the Marine Environment. Since 1998 OP, NP and NP ethoxylates are listed by OSPAR as Chemicals for Priority Action. Another fundamental regulation is the European Water Framework Directive 2000/60/EC. Annex X of this directive lists NP and OP as priority hazardous substances with OP still in the testing process. The aim of these regulations is to ensure, that emissions and losses of these substances to surface waters must be reduced to zero within the next two decades. For an efficient monitoring of these compounds in the environment and verification of the compliance with regulations, a reliable and rugged analytical method is crucial. However, the analysis of alkylphenols and their ethoxylates is challenging, primarily because of the lack of availability of labeled internal standards. Instead, often the n-isomers of OP and NP and their ethoxylates are used as internal standards, which usually leads to erroneous results because the n-isomers exhibit different adsorption and elution properties compared with the branched isomers.
Another particular analytical challenge is caused by NP itself. Due to the production process, technical grade NP comprises a complex mixture of isomers. The GC-MS analysis in Figure 1 shows that the mixture consists mainly of branched para-isomers (>90%) with branched ortho-isomers (<10%) and traces of decylphenol. The para-isomers are further shown to comprise approximately ten distinct compounds. The NP ethoxylates normally display the same pattern of isomers as the technical grade NP they are derived from. One of the most prevalent isomers in technical grade NP is 4-(3,6-dimethyl- 3-heptyl)phenol (363-NP), the structure and MS spectrum of which appear in Figure 1.
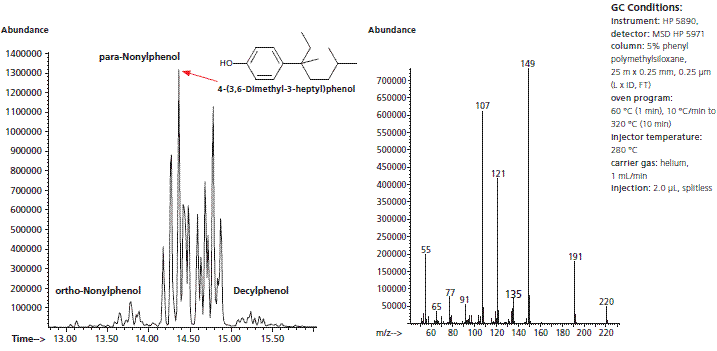
Figure 1. GC-MS of technical grade nonylphenol and detailed mass spectrum of isomer 363-NP
The mono- and diethoxylates of NP behave similarly, with 4-(3,6-dimethyl- 3-heptyl)phenol-monoethoxylate (363-NP1EO) and 4-(3,6-dimethyl- 3-heptyl)phenol-diethoxylate (363-NP2EO) being the major isomers. The high percentage and the favorable fragmentation pattern of these isomers make them ideal internal standards for the analysis of technical grade NP and its ethoxylate derivatives. 13C-ring-labeled and deuterated 363-isomers of NP and its mono- and diethoxylates have been synthesized for this purpose.
The analysis of OP differs from the analysis of NP because it possesses only one significant isomer, 4-(1,1,3,3-tetramethylbutyl)phenol, often called 4-tertoctylphenol, and the resulting ethoxylates. Other isomers do not contribute so the synthesis of internal standards for OP and ethoxylates focuses only on the exact these compounds. Figure 2 is a summary of all isotopically-labeled high-purity internal standards that have been synthesized for the analysis of NP and OP and their ethoxylates described above. The mass difference of 6 amu is ideal for GC-MS. For LC-MS/MS applications, twice-deuterated compounds are available.
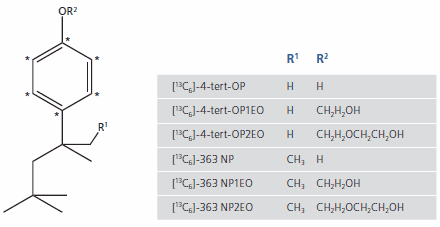
Figure 2. Molecular Structures of 13C-labelled octyl- and nonylphenol and associated ethoxylates. * = 13C
Actually, in Germany the ISO 18857-2 for the analysis of OP, NP and their mono- and diethoxylates, including bisphenol A, in surface water is going to be worked out The method utilizes solid phase extraction (SPE) and quantification by GC-MS after derivatization with MSTFA. Comprehensive studies, which included interlaboratory proficiency testings, proved the suitability of the internal standards listed in Figure 2 and resulted in precise and accurate measurements of the target analytes. As a result, all internal standards so listed were included in ISO 18857-2. For a final evaluation there will be an international interlaboratory proficiency testing in October/ November 2007 in which all interested laboratories are encouraged to participate (for information on participation please contact the authors).
Sigma-Aldrich is pleased to offer the complete range of labeled compounds listed in ISO 18857-2 for the analysis of OP, NP and their mono- and diethoxylates. The corresponding product numbers and concentrations are listed in Table 1.
13C6-labelled
Deuterated
Other products for alkylphenol and alkylphenol ethoxylate analysis
Table 1 Labeled octyl- and nonylphenol and associated ethoxylates from us, solutions in acetone
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?