IgG Antibody Purification with HiTrap® Protein G HP Columns
HiTrap® Protein G HP (Figure 3.10) is a convenient, ready-to-use column prepacked with Protein G Sepharose® High Performance. The columns are available in 1 mL and 5 mL sizes. In common with all HiTrap® columns, HiTrap® Protein G HP can be used for rapid antibody purification with a syringe, pump, or chromatography system. Furthermore, purification capacity can be greatly increased by connecting columns in series.
Figure 3.10.HiTrap® Protein G HP columns are designed for antibody purification using a syringe, pump, or chromatography system.
Figure 3.11. shows the purification of mouse monoclonal IgG1 on HiTrap® Protein G HP. The monoclonal antibody was purified from a hybridoma cell culture supernatant.
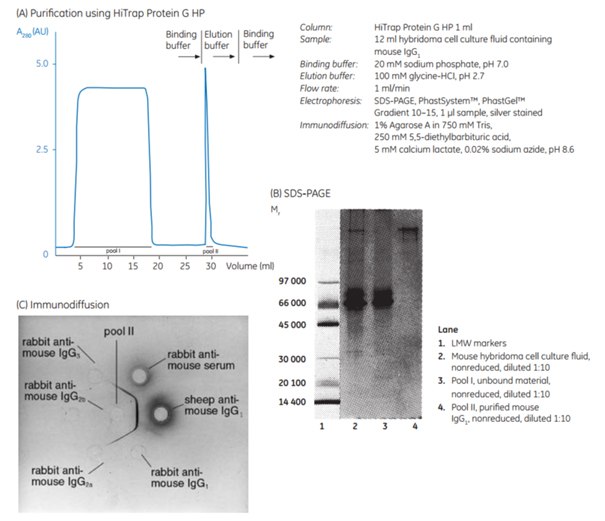
Figure 3.11.(A) Purification of mouse monoclonal IgG1 from cell culture supernatant on HiTrap® Protein G HP 1 mL column. Purity of mouse IgG1 was confirmed by (B) nonreducing SDS-PAGE on PhastSystem using PhastGel 10-15 (silver stained), and (C) agarose-gel immunodiffusion.
Sample Preparation
Refer to Chapter 2 (Desalting and buffer exchange) for general considerations.
The sample should be adjusted to the composition of the binding buffer. This can be done by either diluting the sample with binding buffer or by buffer exchange using HiTrap® Desalting, HiPrep 26/10 Desalting or PD-10 Desalting columns, Chapter 2 (Desalting and buffer exchange).
The sample should be fully solubilized. We recommend centifugation or filtration immediately before loading on the column to remove particulate material (0.45 µM filter).
Never apply turbid solution to the column.
Buffer Preparation
Binding buffer: 0.02 M sodium phosphate, pH 7.0
Elution buffer: 0.1 M glycine-HCl, pH 2.7
Neutralizing buffer: 1 M Tris-HCl, pH 9.0
Water and chemicals used for buffer preparation should be of high purity. Filter buffers through a 0.45 µM filter before use.
For purification using a syringe on HiTrap® Protein G HP 1 mL or 5 mL columns, buffers can be prepared from the 10× stock solutions of binding and elution buffers supplied with Ab Buffer Kit.
Purification
- Prepare collection tubes by adding 60 to 200 µL of 1 M Tris-HCl, pH 9.0 per milliliter of fraction to be collected.
To preserve the activity of acid-labile IgG, we recommend adding 60 to 200 µL of 1 M Tris-HCl pH 9.0 to collection tubes, which ensures that the final pH of the sample will be approximately neutral.
- Fill the syringe or pump tubing with distilled water. Remove the stopper and connect the column to the syringe (use the connector supplied), laboratory pump, or chromatography system “drop to drop” to avoid introducing air into the system.
- Remove the snap-off end at the column outlet.
- Wash out the ethanol with 3 to 5 column volumes of distilled water.
- Equilibrate the column with at least 5 column volumes of binding buffer. Recommended flow rates are 1 mL/min (1 mL column) and 5 mL/min (5 mL column)*.
- Apply the pretreated sample using a syringe fitted to the Luer connector or by pumping it onto the column. For optimal results, use a flow rate of 0.2 to 1 mL/min (1 mL column) and 0.5 to 5 mL/min (5 mL column) during sample application.
- Wash with binding buffer (generally at least 5 to 10 column volumes) until the absorbance reaches a steady baseline or no material remains in the effluent. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for washing.
* 1 mL/min corresponds to approximately 30 drops/min when using a syringe with a 1 mL HiTrap® column; 5 mL/min corresponds to approximately 120 drops/min when using a 5 mL HiTrap® column.
IgG from most species and subclasses bind to protein G at near physiological pH and ionic strength. For the optimum binding conditions for IgG from a particular species, consult the most recent literature.
Avoid excessive washing if the interaction between the antibody and ligand is weak, since this might decrease yield.
- Elute with elution buffer using a one-step or linear gradient. For step elution, 5 column volumes are usually sufficient. For linear gradient elution, 10 to 20 column volumes are usually sufficient. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for elution.
Protein G Sepharose chromatography media bind IgG over a wide pH range with strong affinity at neutral pH. To elute the IgG, it is necessary to lower the pH to between 2.5 and 3.0 depending on the antibody. If biological activity of the antibody or antibody fragment is lost due to the low pH required for elution, try Protein A Sepharose; the elution conditions used are generally milder (Table 3.5).
- After elution, regenerate the column by washing it with 3 to 5 column volumes of binding buffer. The column is now ready for a new purification of the same antibody.
Desalt and/or transfer purified IgG fractions to a suitable buffer using a desalting column (Chapter 2, Desalting and buffer exchange).
To increase capacity, connect several HiTrap® Protein G HP columns (1 mL or 5 mL) in series. HiTrap® columns can be used with a syringe, a peristaltic pump or connected to a liquid chromatography system (Chapter 5 for details of ÄKTA chromatography systems).
Reuse of HiTrap® Protein G Sepharose HP columns depends on the nature of the sample and should only be considered when processing identical samples to avoid cross-contamination.
Storage
Before storage we recommend to wash the column with 5 column volumes of 20% ethanol to prevent microbial growth. Store the column in 20% ethanol at 2 °C to 8 °C.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?