Potassium Organotrifluoroborates
Organotrifluoroborates: Versatile and Stable Boronic Acid Surrogates
Prof. Gary Molander has extensively developed the chemistry of organotrifluoroborates. These bench stable boronic acid surrogates are useful for Suzuki-Miyaura cross-coupling reactions and have also been used for a variety of other C-C bond forming reactions. Importantly, these reagents are compatible with a wide range of functional groups and are stable to many commonly used and harsh reaction conditions.
Benefits of Organtrifluoroborates:
- Bench stable reagents that can be stored indefinitely
- Can be effectively coupled with a wide range of organic electrophiles
- Exist as tetracoordinated monomeric species
- Are less prone to protodeboronation than their boronic acid or boronate ester counterparts
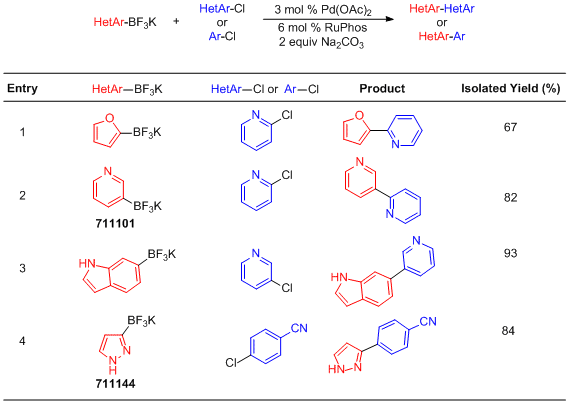
Suzuki-Miyaura Cross-Coupling with Organotrifluoroborates
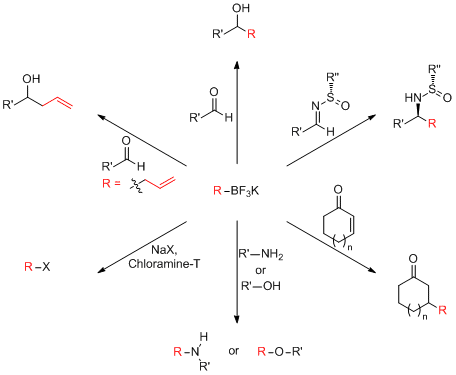
Organotrifluoroborates from us
For a complete list of organotrifluoroborates.
1.
Molander G, Figueroa R. 2005. Aldrichimica Acta.(38):49-56.
2.
Darses S, Genet J. 2003. Potassium Trifluoro(organo)borates: New Perspectives in Organic Chemistry. Eur. J. Org. Chem.. 2003(22):4313-4327. https://doi.org/10.1002/ejoc.200300294
Materials
Loading
登录以继续。
如要继续阅读,请登录或创建帐户。
暂无帐户?