Red-Al® Reducing Agent
Red-Al®, or sodium bis(2-methoxyethoxy)aluminum dihydride (Vitride®, SMEAH), is a versatile reducing agent and a good substitute for LiAlH4 in many reactions. Red-Al® is nonpyrophoric, although still moisture-sensitive, and is available in solution, allowing for easier handling compared to LiAlH4.
Red-Al® is particularly effective at the reduction of epoxides. A recent example employs Red-Al® in a key step in the asymmetric synthesis of (R)-fluoxetine hydrochloride. The enantiomerically pure α,β-epoxyamide was reduced by Red-Al® in the presence of 15-crown-5 to produce the β-hydroxyamide in good yield and excellent selectivity (Scheme 23).1
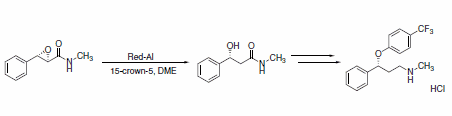
Scheme 23
Cao and co-workers utilized Red-Al® to convert propargyl chlorides to allenes, which were subsequently used in a tandem Pauson-Khand reaction to create 3,7-diisopropylsilyldicyclopenta-[a,e]pentalene, a 14π annulene (Scheme 24).2
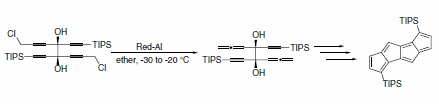
Scheme 24
The preparation of highly functionalized α,β-disubstituted alkenoates can be achieved under mild conditions by reducing an acetylenic ester with Red-Al® and quenching the reaction with iodine to yield the vinyl iodide. The vinyl iodide can be subsequently transformed via Sonogashira or Stille coupling to form the desired α,β-disubstituted alkenoates (Scheme 25).
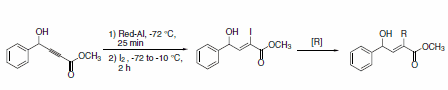
Scheme 25
Red-Al® displays excellent (E)-selectivity in the reduction and does not reduce the methyl ester.3 Red-Al® reduction can selectively produce dialdehydes from aromatic diesters in the presence of N-methylpiperazine (Scheme 26). Although Red-Al® is regularly employed in the reduction of esters to aldehydes, reduction of the diester without the addition of the amine produced only the dicarbinol.4
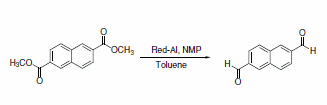
Scheme 26
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?