Dess–Martin Periodinane
Dess-Martin Periodinane (DMP; Product No. 274623, 559873) is a widely used reagent for the mild oxidation of alcohols to aldehydes and ketones.1 The neutral condition of the oxidation reaction makes DMP a suitable choice in syntheses of sensitive, functionally complex intermediates. When used in hydrophilic ionic liquids, DMP oxidations proceed faster than in conventional solvents.2 Other advantages such as selectivity, avoiding the use of toxic (i.e. chromium-based) chemicals, using stoichiometric amounts of reagent, and ease of work-up make DMP a reliable and easy to use oxidizer in organic synthesis.
DMP has been used in a practical and efficient route for the one-step conversion of aromatic and aliphatic aldehydes to acyl azides (Scheme 1). The acyl azides are able to be isolated without rearrangement to the alkyl isocyanate due to the mild reaction conditions.3
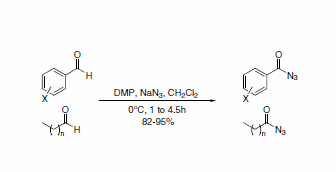
Scheme 1
Intramolecular competition between a dihydropyridine and isoxazolyl alcohols has also been reported. When oxidation was performed using one equivalent of DMP, the keto-isoxazole-dihydropyridine was produced in 93% isolated yield, with no detectable oxidation of the dihydropyridine (Scheme 2).4
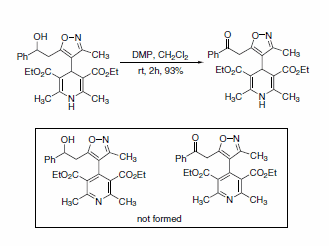
Scheme 2
A report describes a useful procedure for the removal of thioacetals and thioketals using DMP, displaying compatibility with a wide range of functional groups (Scheme 3). A direct route to the corresponding acetals is also possible when the transformation is performed in the presence of an alcohol (Scheme 4).5
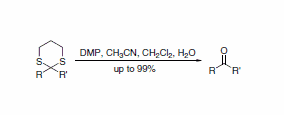
Scheme 3
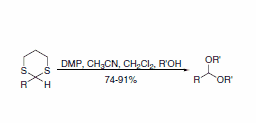
Scheme 4
Rare, complex, and diverse polycycles and heterocycles can be rapidly obtained through DMP-mediated cascade cyclization (Scheme 5).6,7
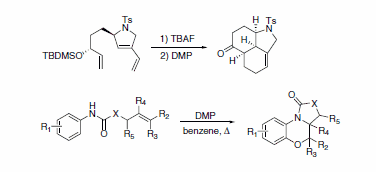
Scheme 5
A slight modification in the reaction conditions allows for the generation of p-quinones from anilides. This approach was used as a key step in concise and efficient syntheses of epoxyquinomycin B (Scheme 6) and topoisomerase-II inhibitor BE-10988 (Scheme 7).8
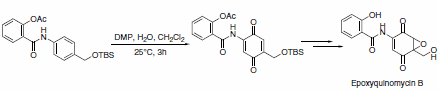
Scheme 6
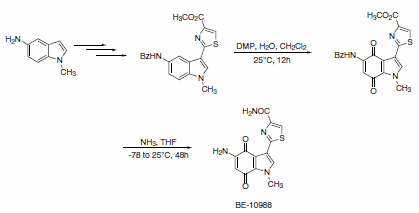
Scheme 7
Dess–Martin periodinane is an effective oxidant for the efficient preparation of epimerization-sensitive, optically active N-protected α-amino aldehydes with high enantiomeric excess (Scheme 8).9
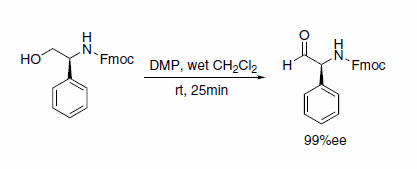
Scheme 8
DMP-mediated oxidation provides a convenient route to the preparation of highly reactive acyl nitroso compounds. These products can be trapped by dienes to form cycloadducts (Scheme 9).10
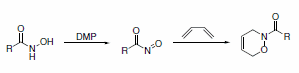
Scheme 9
References
如要继续阅读,请登录或创建帐户。
暂无帐户?