Reducing Organic Photoredox Catalysts for Visible Light-Driven Polymer and Small Molecule Synthesis
Introduction
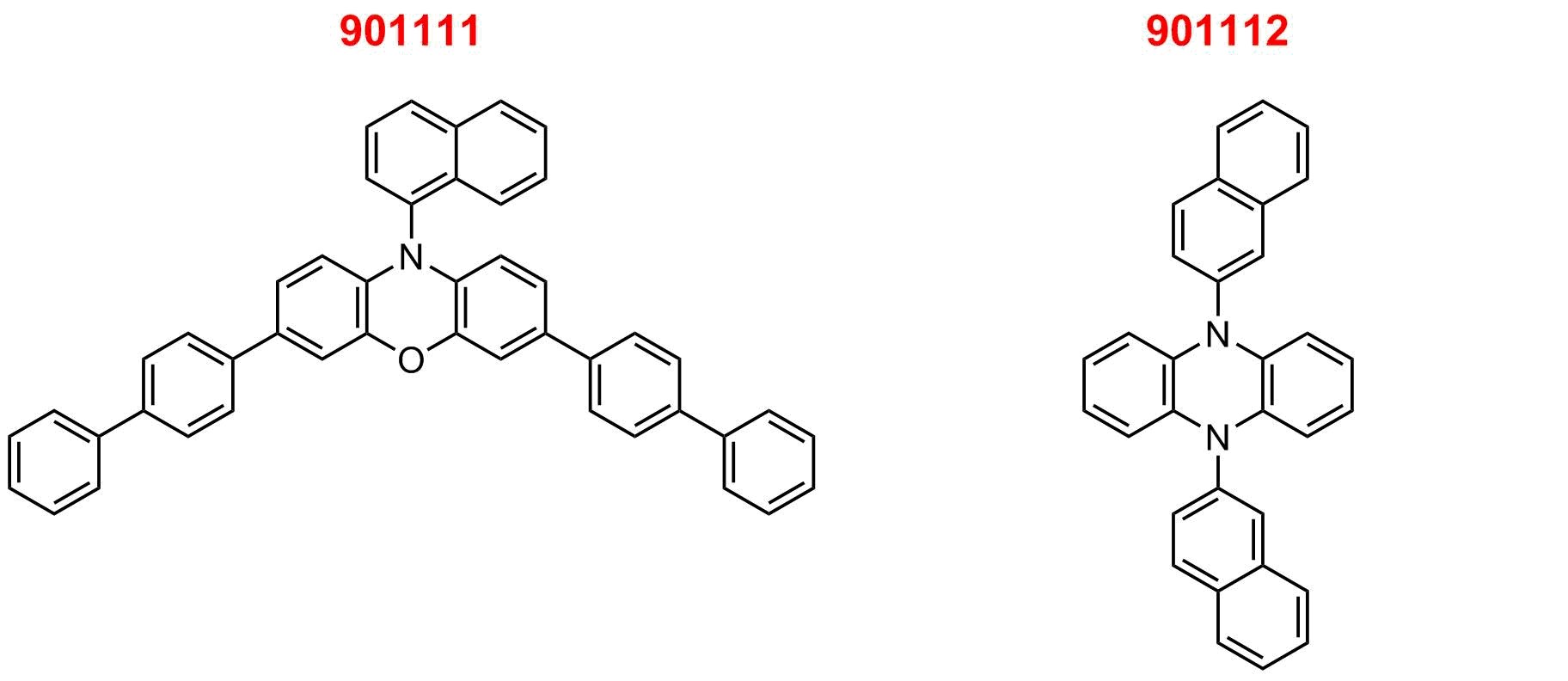
Photoredox catalysis has emerged as a powerful synthetic methodology to form challenging covalent bonds under mild reaction conditions using light irradiation. Recently, phenoxazine (901111) and dihydrophenazine (901112) organic photoredox catalysts were computationally designed and developed to possess photophysical and electrochemical properties pertinent to photoredox catalysis. Specifically, they absorb visible light, have high molar absorptivity, redox reversibility, charge transfer excited states, long triplet excited state lifetimes, and high triplet quantum yields.
Phenoxazine- and dihydrophenazine-derived compounds were demonstrated in the application of photoredox catalyzed atom transfer radical polymerization (ATRP) for controlled polymer synthesis and small molecule transformations such as trifluoromethylation and dual photoredox/nickel catalyzed C-N and C-S cross-couplings. In collaboration with the Garret Miyake group, we now offersphenoxazine and dihydrophenazine organic photoredox catalysts.
| E0 (2PC•+/3PC*) = -1.80 V vs. SCE | E0 (2PC•+/3PC*) = -1.80 V vs. SCE | |
| E0 (2PC•+/1PC) = 0.65 V vs. SCE | E0 (2PC•+/1PC) = 0.21 V vs. SCE | |
| Etriplet = 2.45 V | Etriplet = 1.90 V | |
| λmax,abs = 388nm (ε = 26600 M-1cm-1) | λmax,abs = 343nm (ε = 5950 M-1cm-1) | |
| 𝜏triplet = 480 µs (Φtriplet = 90 %) | 𝜏triplet = 480 µs (Φtriplet = 2 %) |
Advantages
- Cost-effective and sustainable alternatives to precious metal complexes
- Highly reducing excited states
- Exhibiting properties important for photoredox catalysis: visible light absorption, high molar absorptivity, redox reversibility, charge transfer excited states, long triplet excited state lifetimes, and high triplet quantum yield
Applications

Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?