PEPPSI™-IPent for Demanding Cross-Coupling Reactions
Introduction
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions. PEPPSI™ palladium precatalysts have become a choice alternative to palladium phosphine complexes, as they are comprised of a bulky N-heterocyclic-carbene (NHC) ligand bonded to palladium and a s-donating 3-chloropyridine ligand for stability. The PEPPSI™-IPent (732117) further advances the agility of cross-coupling reactions by being air-stable and thus increasing user-friendliness. We are proud to offer the most active of the PEPPSI™ series, PEPPSI™-IPent, in collaboration with the Organ Research Group.1
Advantages
- PEPPSI™-IPent is bench stable: no need for a glovebox
- Poisoning of Pd has not been observed
- Higher pyrrole-based cross-coupling reactions success
- Consistently superior coupling yields over PEPPSI™-IPr
- Effective and versatile catalyst for difficult, sterically-demanding reactions2
Representative Applications
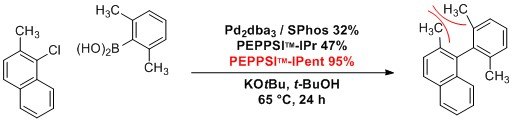
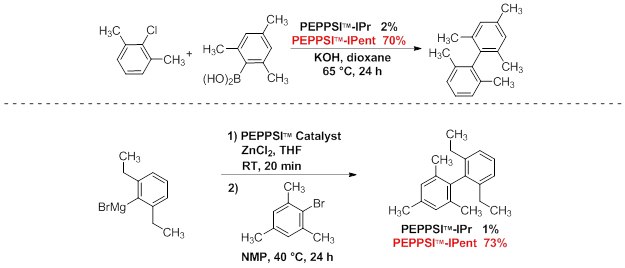
Under relatively mild conditions, PEPPSI™-IPent is a highly effective catalyst in challenging Suzuki–Miyaura and Negishi cross-coupling reactions. This produces tetra-ortho-substituted (hetero)biaryl compounds in high yields, especially when compared to the use of PEPPSI™-IPr. Bulky and acidic substituents were observed to be well tolerated.1,2
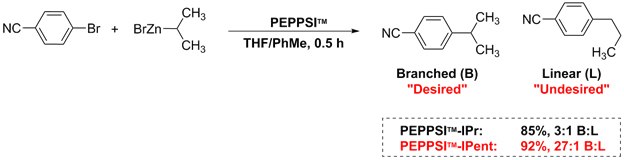
Negishi cross-coupling reactions of secondary alkyl zinc halides with aryl halides utilize PEPPSI™-IPent, boasting higher selectivity than PEPPSI™-IPr. The desired branched product was obtained in higher yields than that of the linear product.1,3
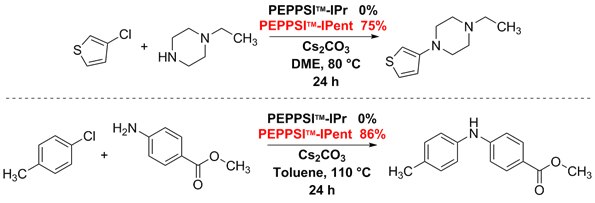
Buchwald–Hartwig–Yagupol’skii aryl aminations typically use strong bases to aid the rate-limiting step, creating conditions that functional groups poorly tolerate. In contrast, using mildly basic conditions and PEPPSI™-IPent, a variety of demanding aryl chlorides were successfully coupled to secondary amines. In addition, PEPPSI™-IPent facilitated the coupling of electron-deficient anilines with electron-rich aryl chlorides. In these reactions, the highly reactive PEPPSI™-IPent consistently outperformed PEPPSI™-IPr.1,4,5
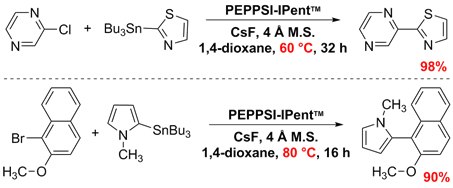
In Stille–Migita cross-couplings of heteroaryl stannanes with aryl halides, PEPPSI™-IPent allowed for lower reaction temperatures below 100 ºC for thermally sensitive cross-coupling partners. Organ and co-workers observed successful coupling within in the range of 30–80 ºC while maintaining consistent yields.1,6
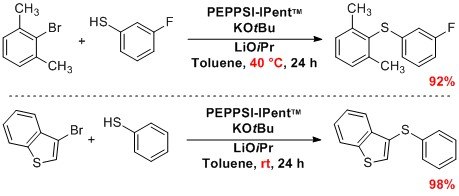
High temperatures frequently used for carbon-sulfur bond formation were circumvented with PEPPSI™-IPent, featuring mild conditions for the formation of thiol ethers by metal-catalyzed sulfinations. Successful reactions have included the sulfination of (hetero)aryl halides with aryl sulfides, alkyl thiols, and also sterically hindered substrates.1,7
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?