X-tremeGENE™ siRNA Transfection Reagent
Introduction
Small inhibitory RNAs (siRNAs) have become the focus of interest in many laboratories. For the first time, these molecules offer an easy way to knock down the expression of selected genes in mammalian cells without having to resort to classical gene knockout techniques.
The process of siRNA-mediated gene silencing is evolutionarily highly conserved. Probably all higher eukaryotes contain an enzyme system (Dicer RNase) that is required to process double-stranded RNAs (dsRNA) to small “guidance” molecules. The resulting short 21- to 24-bp double-stranded RNA (siRNA) activates a cellular RNase system (RISC complex) that degrades any mRNA with complementary sequence to the siRNA. A break-through development for the application of RNA interference (RNAi) in mammalian cells was the discovery that direct introduction of short 21-bp dsRNA oligonucleotides into cells mediates effective gene silencing but does not trigger the so-called interferon response. This mechanism is present in mammalian cells as a defense against viruses and shuts down protein synthesis in reaction to the presence of any long dsRNA, irrespective of its sequence1.
The practical application of RNAi in the laboratory therefore requires a method that allows the effective introduction of short dsRNA molecules into mammalian cells. An additional obstacle to overcome is the need for optimization of the siRNA sequence. Not every 21-bp dsRNA shows the same efficiency in silencing the corresponding mRNA. Despite the availability of sophisticated siRNA computer design tools, a certain amount of actual bench testing is still necessary. On many occasions, there is strong demand for a test system that is simple to manipulate and allows quick pre-testing of potential RNAi oligonucleotides for their knock-down efficiency towards the protein of interest. The simultaneous transfection of siRNA and an expression plasmid for the protein to be silenced offers a unique solution to this practical problem. An additional benefit of this system is that silencing can be detected at the protein level even if no antibody against the target is available. A large variety of epitope tags can be fused to the cDNA corresponding to the target gene if it is expressed ectopically from a vector. Moreover, this approach also allows an assessment of the siRNA specificity, which needs to be checked in every RNAi experiment. Possible off-target effects can be revealed by including an expression vector for a target protein that carries a mutation in the siRNA recognition sequence.
Since classical calcium phosphate transfection methods are very inefficient for the transfection of small dsRNA molecules, a series of newly developed lipofection reagents were tested for their efficacy in delivering DNA as well as siRNA into cells.
Materials and Methods
One day prior to transfection, the human embryonic kidney cell line 293T was plated in 4ml DMEM (4.5 g/L glucose, supplemented with 10% FCS and penicillin/streptomycin) into 6-well plates at a density of 1x106 cells/well. Following over-night incubation, the medium was changed to 2ml of antibiotic-free DMEM/FCS.
All siRNAs used were chemically synthesized by Dharmacon Inc. For transfection with X-tremeGENE™ siRNA Transfection Reagent, 0.6 ug expression vector (based on a CMV promoter) and 6.25 ul of a 20-uM solution of either a specific siRNA duplex or a control siRNA directed against the nuclear protein Lamin C were diluted in 125 ul serum-free DMEM (total nucleic acid content 2.5 ug). In parallel, 25 ul X-tremeGENE™ siRNA Transfection Reagent was added to 100 ul serum-free DMEM, and the resulting mixture was combined immediately with the diluted nucleic acids. After 20 minutes incubation at room temperature, the solution was added dropwise to the cells. Without any further medium change, the cells were assessed for citality by microscopic inspection 24 hours after lipofection and subsequently lysed for protein analysis by immunoblot according to standard methods. Control reactions employing commonly used lipofection reagents of other suppliers were done exactly according to the instructions of the manufacturers.
Results and Discussion
Even after 24 hours of continuous contact with the cells, none of the reagents had caused significant mortality in the transfected cell population. As judged by specific repression of protein expression, however, only reagents from supplier A and X-tremeGENE™ siRNA Transfection Reagent were capable of delivering DNA and siRNA simultaneously into the cells (Figure 1).
With reagent from supplier B protein expression was detected, but the RNAi effect was completely absent, indicating that RNA is not efficiently transfected with this compound. Lipofection component from supplier C was completely ineffective with the DNA/RNA mixture. In contrast, a clear inhibition of the target gene expression by specific siRNA was seen after lipofection with the reagent from supplier A and X-tremeGENE™ siRNA Transfection Reagent. In a direct comparison between two liposome formulations, X-tremeGENE™ siRNA Transfection Reagent seems to be superior. More protein is expressed after transfection with the X-tremeGENE™ siRNA Transfection Reagent, showing the high transfection efficiency that can be obtained with this compound. Because a better transfection rate allows detection of proteins with fewer cells, this type of assay can be done with X-tremeGENE™ siRNA Transfection Reagent in much smaller well volumes, thus making it possible to run these experiments in a more high-throughput format.
As judged by the repression ration reached with specific versus control siRNA, the higher transfection efficacy of X-tremeGENE™ siRNA Transfection Reagent compared with the reagent from supplier A is not limited to transport of RNA or DNA alone. This is important because a preferential transfection of only one nucleic acid type might greatly contort the observed RNAi effect and therefore lead to a misjudgment of the underlying inhibitory efficiency. In summary, X-tremeGENE™ siRNA Transfection Reagent is a superior new reagent for any transfection requirement in conjunction with siRNA.
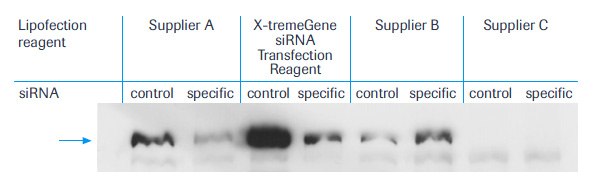
Figure 1:Typical example of an immunoblot. Cells were co-transfected with 0.6 ug expression vector for FLAG-tagged protein and 6.25 µl of a 20 µM solution of either a specific siRNA a control siRNA directed against IamC as indicated. The arrow denotes the expected position of the FLAG-tagged protein. Equal amounts of cellular proteins (20 µg/lane) were applied. Reagents were used as noted.
Materials
References
For life science research use only.
Not for use in diagnostic procedures.
如要继续阅读,请登录或创建帐户。
暂无帐户?