Scepter™ 3.0 Cell Counter – How it Works
Why automate your cell counting?
For decades, hemocytometry was the gold standard for cell counting. A sample of media or buffer containing cells may or may not be mixed with a dye. A precise volume (usually 10 µL) of this mixture is pipetted into a groove in the hemocytometer, where it fills a chamber whose floor has a grid precisely etched onto its surface. One or more small or large square fractions of the grid are counted, and, depending on which area has been counted, a scaleup calculation is performed to return a concentration of cells per milliliter.
So what’s the problem with using a hemocytometer?
Automated cell counters eliminate steps that are vulnerable to human error at virtually every step:
- If a viability dye is used, the dye volume and the sample volume must be precise for the count to be valid, and the operator must remember to factor this dilution into the cell density calculation
- The hemocytometer chamber must be filled carefully and completely for the measurement to be meaningful
- Hemocytometry relies on subjective visual recognition of a cell by the human operator
- Each grid region must be accurately tallied by visual enumeration by the technician
- The technician must sample multiple areas of the grid and average these counts accurately to ensure precision
- The operator must understand and correctly apply the hemocytometer calculation method based on the size of the area counted
- The technician must correctly transcribe the calculated count into lab notebook or other lab record
Faster Cell Counts
Scepter™ counting is 7 to 10 times faster than hemocytometry-and also faster than other automated counters.
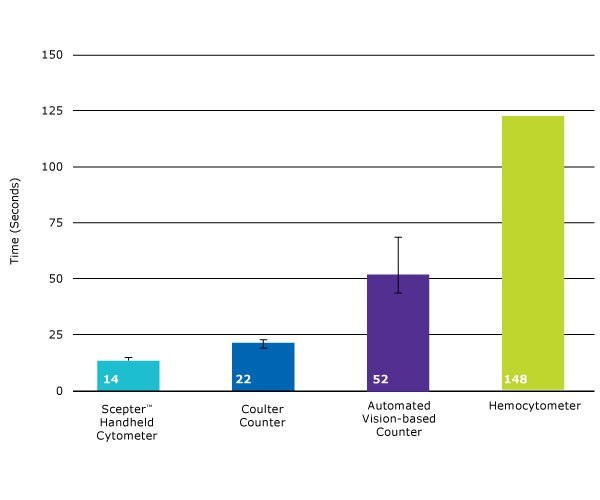
Figure 1.The time required to perform cell counts using various methods was compared using a sample concentration of 500,000 cells/mL. Scepter™ counting (14 seconds on average, using the 60 μm sensor) is significantly faster than other counting methods. Using the 40 μm sensor, Scepter™ counts are complete within 25 seconds, on average (data not shown).
The Gold Standard for Cell Counting: Coulter Impedance
The Scepter™ 3.0 cell counter uses the Coulter principle of impedance-based particle detection to reliably and accurately count every cell in your sample. Coulter impedance is more precise than hemocytometry and automated vision-based counting, demonstrating smaller average coefficients of variation when compared with these methods. Here’s how it works:
- Precise volumes are drawn into the Scepter'"' sensor.
- As cells flow through the aperture in the sensor, resistance increases, and, by Ohm's law (V=IR, where V=voltage, I=current, and R=resistance), this increase in resistance causes a subsequent increase in voltage.
- Voltage changes are recorded as spikes with each passing cell.
- Spikes of the same size are bucketed into a histogram and counted.
- This histogram gives you quantitative data on cell morphology that can be used to evaluate the quality and health of your cell culture.
Precise Counts Come from Larger Sample Size
As with other technologies that are based on flow cytometry (where cells pass one-by-one through a fluidic channel past a detector), precise data is enabled by the large sample sizes that visual counters can’t achieve because they rely on a scatter of cells spread onto a single microscopic field. The Scepter™ 3.0 counter returns histogram data from thousands of cells per sample, resulting in more precise counts
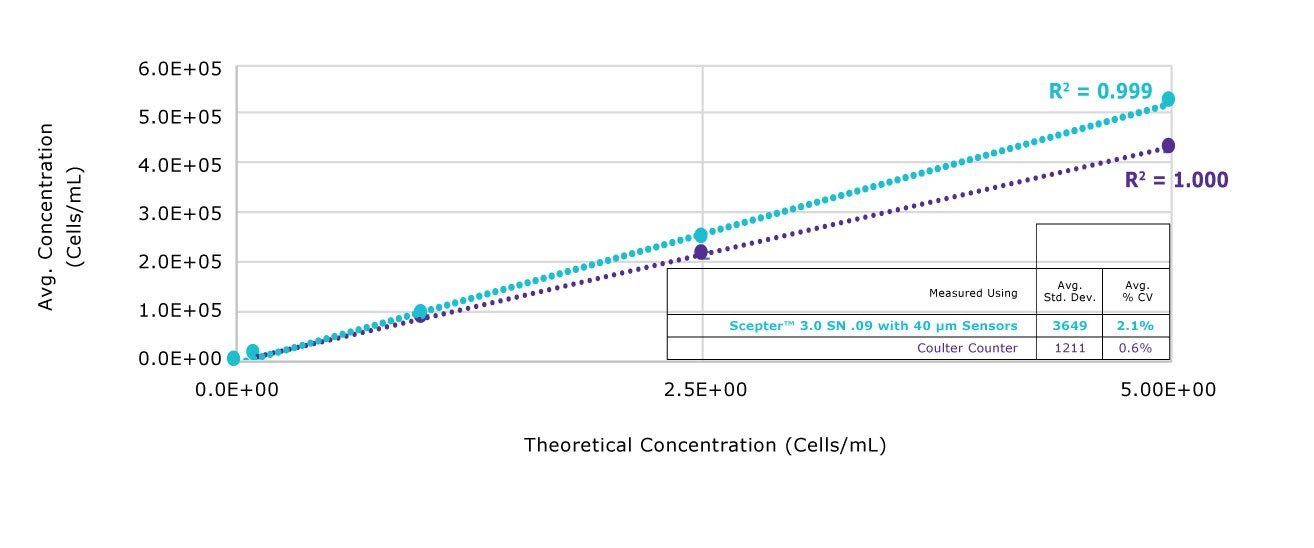
Figure 2.A range of CHO cell concentrations were measured using the Scepter™ 3.0 counter equipped with the 60 μm sensor, and compared with the same samples measured using the Coulter Z2 with 100 μm aperture. Measurement of concentration was highly linear across concentrations ranging from 100,000 to 500,000 cells/mL using both methods.

Figure 3.Jurkat cells were measured to test accuracy and reproducibility of cell size measurements using the Scepter™ 3.0 Cell Counter with both 40 μm and 60 μm sensors. Results are compared to the same measurement obtained with a Coulter® Counter Z2™ Instrument equipped with 100 μm aperture. Data are from five measurements per sample.
Some automated counters require cells to be in PBS, which can handicap productivity by requiring a buffer exchange. To be an efficient part of cell culture maintenance and experiment prep, a cell counting method should also be able to accommodate cells where they live: in media, with or without FBS supplementation.
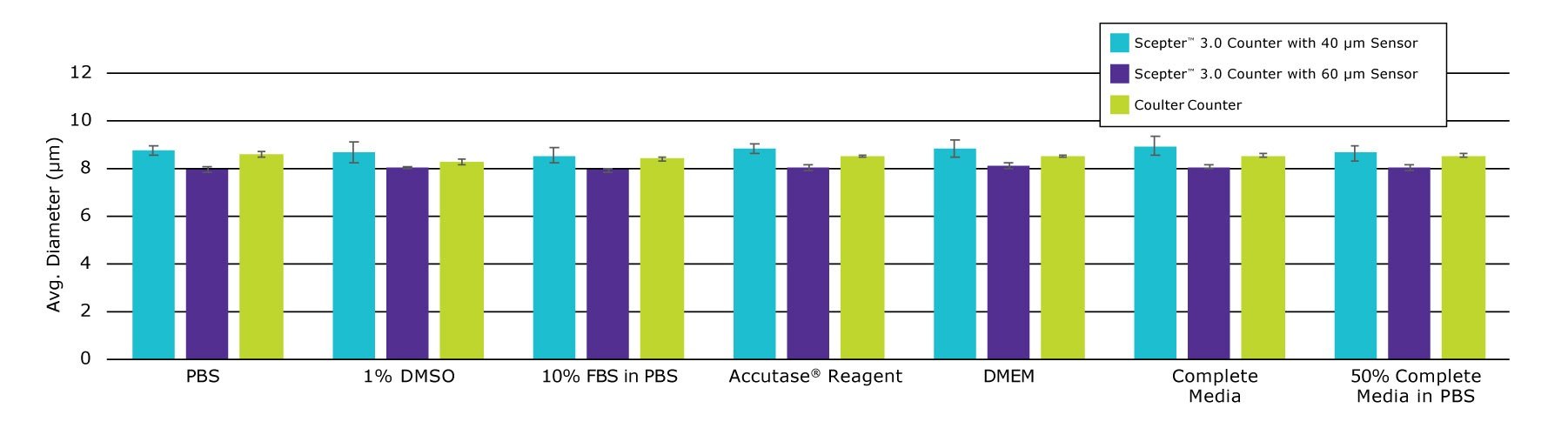
Figure 4.Polystyrene beads of known diameter (8 μm) suspended in various cell culture buffers and reagents were measured using the Scepter™ 3.0 Cell Counter with both 40 μm and 60 μm sensors. Results are compared to the same counts obtained with a Coulter® Counter Z2™ Instrument equipped with 100 μm aperture. Data are from three measurements per sample.
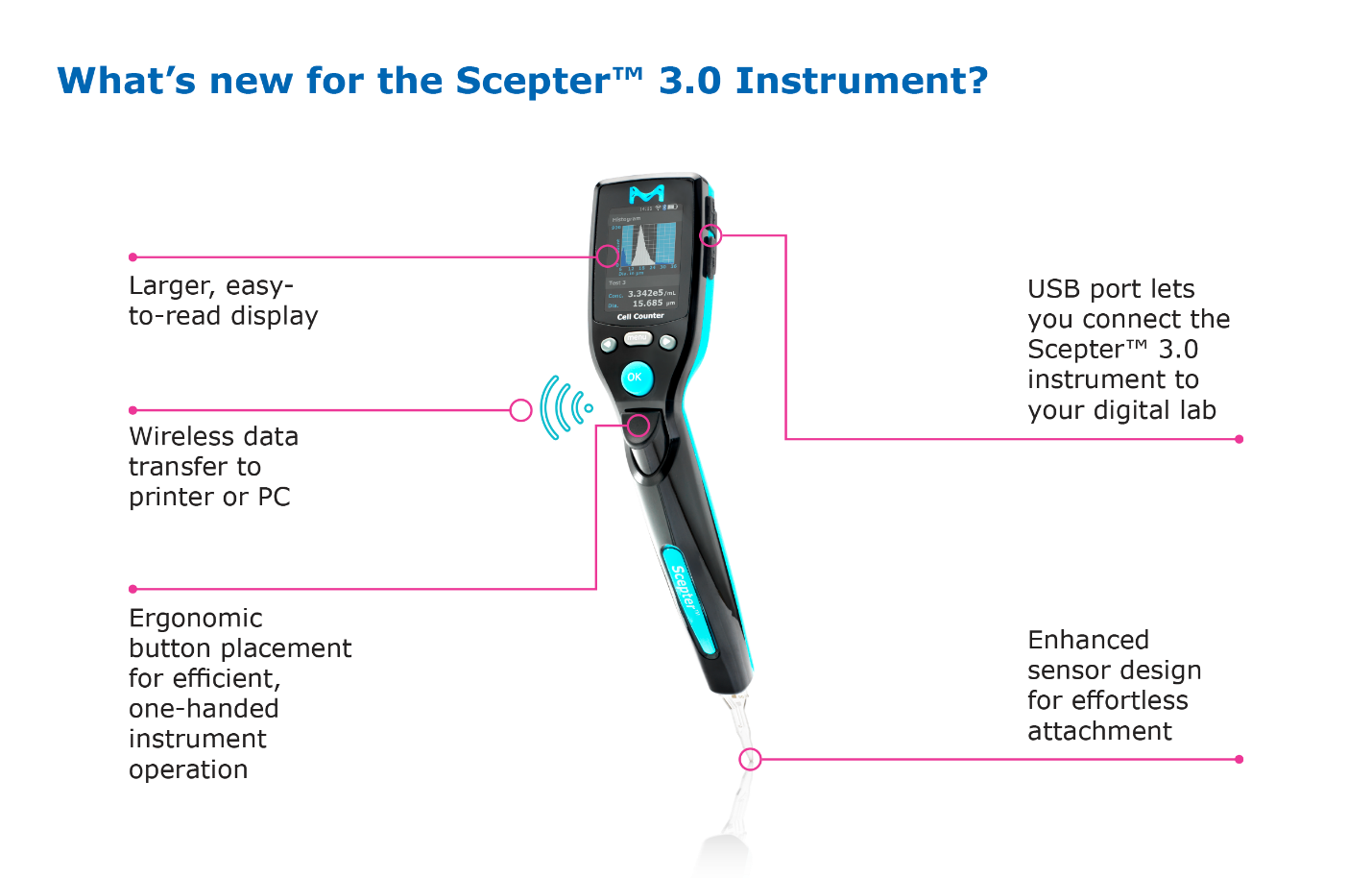
- The instrument is even more streamlined with ergonomic control placement, but the larger readout makes it easier to follow prompts and see results
- The charging station that keeps the Scepter™ ready when your cells are now mounts on the tissue culture hood, or anywhere else that keeps it at hand where you need it.
- Perhaps surprisingly, the microfluidic Coulter-based technology that gives the Scepter™ 3.0 its accuracy is actually housed in the sensor tip. Despite the sophistication of this technology already packed into a small space, we re-engineered the sensor to achieve a more streamlined shape in two dimensions that not only uses less material, but makes it easier to sample cells directly from microcentrifuge tubes and plate wells.
Sample Preparation and Counting Process
Sample preparation
Start with a single-cell suspension, diluted to a total volume of 100 μL (recommended) to a concentration of 10,000 to 500,000 cells/mL in a microcentrifuge tube or plate well.
Counting process
- Turn on the Scepter™ cytometer by pressing the control button on the back of the instrument and wait for on-screen instructions to appear.
- When prompted, attach a sensor to the end of the Scepter™ unit with the electrode sensing panel facing toward the front of the instrument, and you'll see detailed instructions for each step of the counting process.
- Pipette once to draw sample into the sensor. 50 μL of your cell suspension is drawn into the microfabricated, precision-engineered channel embedded in the sensor. The cell sensing zone detects each cell drawn into the sensor and thus cell concentration is calculated. The sensing zone also measures cell sizes and cell volumes with sub-micron and sub-picoliter resolution, enabling the Scepter™ cytometer to display a histogram distribution of cell size or cell volume.
- Precise volumes are drawn into the Scepter™ sensor.
- As cells flow through the aperture in the sensor, resistance increases; and, by Ohm's law (V=IR, where V=voltage, 1=current, and R=resistance), this increase in resistance causes a subsequent increase in voltage.
- Voltage changes are recorded as spikes with each passing cell.
- Spikes of the same size are bucketed into a histogram and counted.
- This histogram gives you quantitative data on cell morphology that can be used to evaluate the quality and health of your cell culture.
For a more detailed explanation of the counting process, watch our Scepter™ 3.0 demonstration video.
Materials Scepter™ 3.0 Handheld Automated Cell Counter
如要继续阅读,请登录或创建帐户。
暂无帐户?