Derivatization and Separation of Aliphatic Amines
The aliphatic amines are commonly analyzed in environment and biological samples.1 Detection and separation of aliphatic amines by HPLC has been reported2,3,4 earlier. However, aliphatic amines, generally, do not show ultraviolet absorption or emit fluorescent light, and this makes their detection difficult. The derivatization allows quantitative conversion of amines to a detectable derivative, with minimal side reactions under mild conditions. Huang et al.1 recently reported that the aliphatic and diamines can be conveniently separated by HPLC following a pre-column derivatization with 2,6-dimethyl-4-quinolinecarboxylic acid N-hydroxysuccinimide ester (DMQC-OSu). The DMQC-OSu reagent readily reacts with primary and diamines exhibiting several advantages; such as, good selectivity in aqueous solution, fewer by-products, mild reaction conditions and excellent derivative stability. The reaction is illustrated in Figure 1. Its use as a spectrofluorometric reagents has also been reported by Cao et al.5
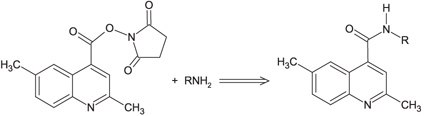
Figure 1.DMQC-OSu reaction with aliphatic amine.1
Huang et al..1 reported a method for simultaneous analysis of aliphatic and diamines using reversed-phase HPLC with a C18 column, after pre-column derivatization with DMQC-OSu. Following this procedure, they reported good separation of various primary amines and diamines with improved sensitivity and selectivity. Our study presents results of derivatizing aliphatic amines with DMQC-OSu following the method of Huang et al..1 Derivatized analyte was evaluated by Ascentis® Express HPLC column with expectation of reducing run time and improving peak shape.
A mixed aqueous amine solution was used that contained a mixture of 8 different aliphatic amines (methyle-, ethyl-, propyl-, ethylenedi-, butyl-, amyl-, hexyl- and heptylamines). The solution was derivatized following the method reported by Huang et al..1 The derivatization conditions for this study; such as, concentration of DMQC-OSu, buffer pH, reaction temperature and time were, the same optimized conditions as Huang et al..1 used for maximizing derivatization yield. A 20 µL sample of amine solution was mixed with 20 µL of derivatization reagent solution (2 mmole DMQCOSu (Cat. No 49558) in acetonitrile) and 200 µL of 0.2 M boric acid titrate buffered to pH 7.5 with sodium hydroxide at 50 °C for 40 minutes. The mixture was then cooled to room temperature. A sample of 20 µL of this derivatized solution was then injected for HPLC analysis.
An Ascentis Express column of 10 cm length and 2.7 µm particle size was selected for this study compared to the column length of 15 cm and particle size of 5 µm used by Huang et al..1 The derivatized sample was run by HPLC-FL and experiments were performed by varying gradient time for ACD LC simulator. Simulations were run to develop this test method and simulation conditions were confirmed. The chromatogram is shown in Figure 2.
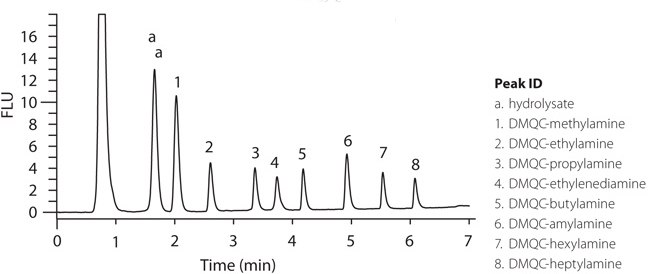
Figure 2.Chromatogram of DMQC-OSu derivatives of amines using Ascentis Express C18 column.
Huang et al.1 suggested that in an amino acid matrix, DMQC-OSu can react with amino acids, and the retention times of amino acid derivatives were found to be shorter than those of aliphatic amine derivatives under the selected chromatographic conditions. Any interference from amino compounds was ruled out in detection of aliphatic amines. Alcohols were were also reported to show no interference with aliphatic amines.1 Consequently, this approach offers high selectivity for analysis of aliphatic amines.
A typical chromatogram from the study of Huang et al.1 showed that under the defined optimum conditions the eight analytes were separated in 16 minutes. Following their optimized derivatization method and similar test conditions, the run time on Ascentis Express column was achieved in seven minutes with reduced re-equilibration from 30 to 20 minutes at equivalent column volumes. Results from the Ascentis Express column show higher peak resolution, especially early in the chromatogram, and improved peak shapes. These results confirm the suitability of the derivatization reagent, 2,6-Dimethyl-4-quinolonecarboxylic acid N-hydroxy succinimide ester and the optimized derivatization method reported by Huang et al..1 Also, the Ascentis Express C18 column allows efficient separation of derivatized primary and secondary aliphatic amines after pre-column derivatization.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?