Two-step Purification of Mouse Monoclonal IgG1 using HiTrap® Protein G HP for the Capture Step
This case shows a purification method for mouse monoclonal IgG from cell culture supernatant using HiTrap Protein G HP for the initial capture. Polishing was performed in the second, SEC step on HiLoad 16/600 Superdex 200 pg. The capture and polishing steps were performed on ÄKTAprime plus. Monoclonal mouse IgG1 was captured in the first step and eluted using a low pH buffer.
Target molecule: Mouse monoclonal IgG1.
Source material: Cell culture supernatant.
Extraction and clarification: Cell culture supernatant filtered through a 0.45 µm filter.
Products
Capture
Binding buffer: 20 mM potassium phosphate, pH 7.0
Elution buffer: 100 mM glycine-HCl, pH 2.7
- Equilibrate column with 5 column volumes of binding buffer.
- Apply sample.
- Wash the column with 10 column volumes binding buffer or until the absorbance at 280 nm has returned to baseline.
- Elute with 5 to 10 column volumes of elution buffer.
- Re-equilibrate with 5 column volumes of binding buffer.
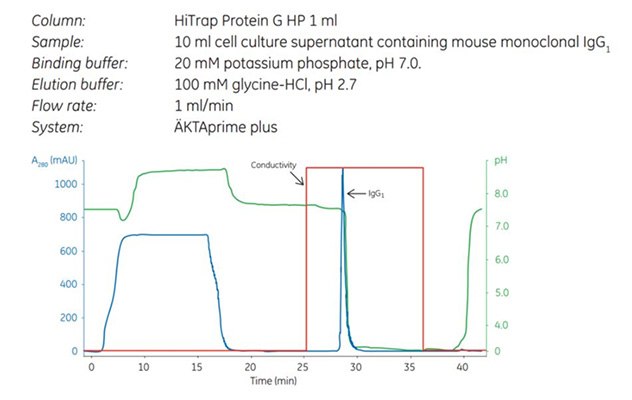
Figure 1.Capture step in a two-step purification of mouse monoclonal IgG1 using HiTrap Protein G HP. The curves shown are absorbance (blue), pH (green), and conductivity (red).
Intermediate Purification
No intermediate step was required as the high selectivity of the capture step gave a sufficiently high level of purity so that only a final polishing step was necessary.
- Equilibrate the column with phosphate buffered saline, pH 7.4 (see Table A3.1, Appendix 3 - Immunoprecipitation techniques).
- Apply sample (maximum sample volume 1% to 2% of total column volume).
- Elute sample in one column volume of buffer. Collect fractions.
- Wash with 2 to 3 column volumes of buffer.
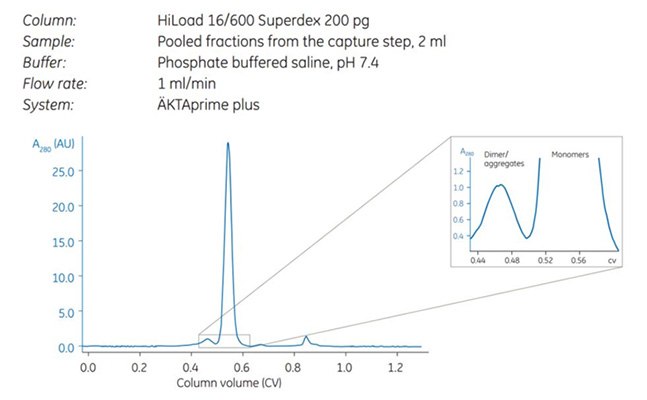
Figure 2.Note the separation between dimers and monomers (magnified).
Affinity purification reduces sample volume and concentrates the sample. SEC is the slowest of all chromatography techniques and the size of the column determines the volume of sample that can be applied. Therefore, it is most logical to use SEC after techniques that reduce sample volume.
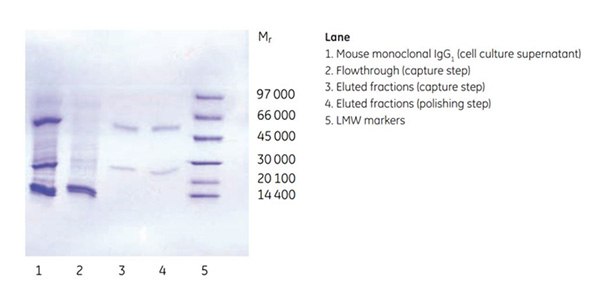
Figure 3.Purity analysis of mouse monoclonal IgG1 by SDS-PAGE, reducing conditions.
Purity was controlled by SDS-PAGE under reducing conditions, which showed that the antibody was highly pure already after the first affinity step. The SEC step further improved target quality by separating the dimer and monomer of the antibody. Note that both dimers and monomers run as heavy and light chains under the reducing conditions used.
如要继续阅读,请登录或创建帐户。
暂无帐户?