Cancer Stem Cell Tumorsphere Formation Protocol
Solid tumors grow in a three-dimensional (3D) spatial conformation, resulting in a heterogeneous exposure to oxygen and nutrients as well as to other physical and chemical stresses. To mimic the 3D spatial conformation, 3D in vitro culture models have been used in cancer research. Cancer stem cells (CSCs) are defined as a small subset of cells within a tumor with the ability to self-renew and often drive tumor progression and recurrence after chemotherapy treatment. Traditionally, cancer stem cells have been isolated from cancer cell lines and tumor biopsies and grown in 3D tumorsphere suspension cultures.
Read more about
Overview of the Tumorsphere Formation Protocol
Expand for more protocol details.
Count Cells
Seed Cells at 1x104 cells/ml
Incubate 4-10 Days
In a Low Attachment Plate
Transfer Spheroids
Transfer Spheroids into a 15 ml Tube
Allow to Settle by Gravity for 10 Minutes
Aspirate Media Leaving Spheroids
Wash Spheroids
Wash Spheroids with 1X PBS
Add Trypsin
Add 1 ml of Trypsin for 2-4 Minutes
Break Apart Spheroids
Break Apart Spheroids by Agitating 10-20 Times Using a Pipet
Count Cells
Seed Cells at 1X104 cells/ml
Tumorsphere Formation Assay
- Detach the cells of a cancer stem cell-containing adherently growing cancer cell line using Trypsin-EDTA Solution (T3924). The cells should be 80–90% confluent and in good condition.
- Centrifuge the cell suspension for 5 minutes at 300 x g and aspirate the supernatant. Resuspend the cells in a small volume, e.g. 3–5 mL, of the 3dGRO™ Spheroid Medium (S3077).
- Count the cells (PHCC360KIT) and adjust the volume with 3dGRO™ Spheroid Medium to obtain a concentration of 1X106 cells/mL.
- Seed the cells in appropriate suspension culture vessels at 1X104 cells/mL, e.g. 40,000 cells in 4 ml of 3dGRO™ Spheroid Medium in each well of a Corning® Costar® Ultra-Low attachment multiwell plate (CLS3471).
- Incubate the culture for 4–10 days, depending on the cell type used. Add one-half of the culture volume of fresh 3dGRO™ Spheroid Medium every 3–4 days. Do not change all the media at once like traditional 2D cell culture.
- Passage the tumorsphere culture before they start to develop a dark center. The optimal passage should be done after 4-10 days depending on the cell type used.
- Collect the tumorspheres by transferring the cells and media into 15 mL conical tubes using a serological pipet.
- Allow the spheres to settle by gravity sedimentation for 10 minutes at room temperature. Aspirate the supernatant, but leave approximately 200 μL in the conical tube. Be careful not to aspirate the tumorspheres.
- Repeat the step 7-8 with an equal volume of PBS (D8662). Gently aspirate the PBS leaving approximately 200 μL in the conical tube.
- Add 1 mL of Trypsin-EDTA (T3924) solution to the tumorspheres and incubate for 2–4 minutes at room temperature. Keep the spheres resuspended in the trypsin solution by pipetting up and down once every 30 seconds. Avoid sedimentation of the spheres.
Note: The optimal incubation time required to achieve complete dissociation must be determined empirically by the user for each cell type. While 2–3 minutes will be optimal in most cases, tumorspheres of some cell types, e.g. MCF-7, may need longer incubation, especially in higher passages. If a completely defined dissociation process is preferred, a recombinant trypsin (T3449) solution may be used as an alternative dissociation reagent according to the supplier`s instructions. - Pipet the spheres up and down 10–20 times using a 1000 μL pipet tip to generate a single cell suspension. Aspirate the cell suspension as normal but tilt the pipet tip slightly at the bottom of the tube when expelling the cells. The shear forces generated facilitate the breakup of any residual cell aggregates. Perform a visual check to confirm that no large cell aggregates remain. Immediately after trituration, add twice the volume of Trypsin Neutralization Solution (T6414) or media.
Note: Do not over-triturate as cell viability will be compromised. Non-dissociated cell aggregates may be removed by passing the cell suspension through a 40 μM cell strainer. - Determine the cell number and viability. Centrifuge the cells for 5 minutes at 300 x g. Discard the supernatant and resuspend the cells in fresh 3dGRO™ Spheroid Medium at 1X106 cells/ml.
- Reseed the cells at 1X104 cells/mL in new Corning® Costar® Ultra-Low attachment multiwell plate (CLS3471). Typically, 6-well plates with 4X104 cells in 4 mL of medium per well are used.
Typical Results
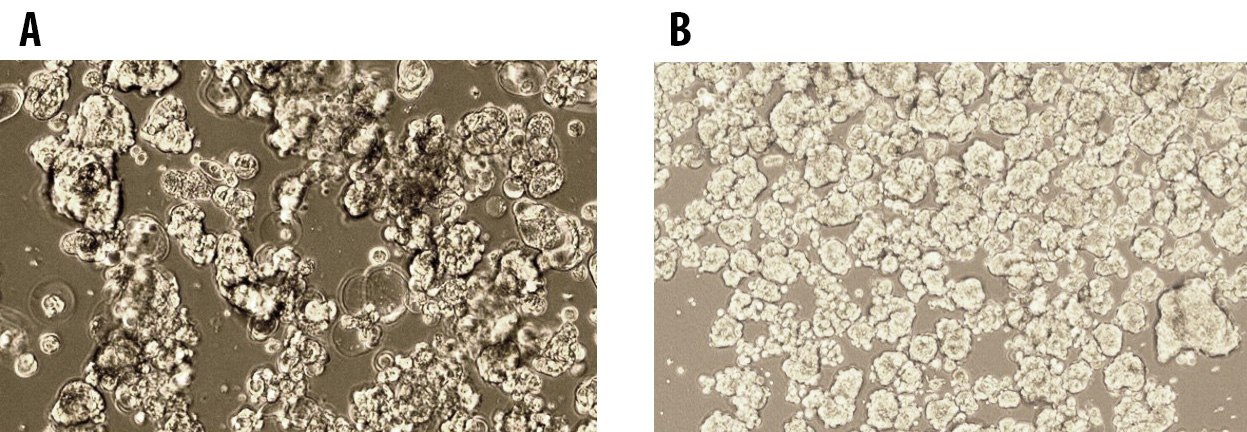
Figure 1. Tumorsphere formation of MCF7 Breast Cancer Cells at Passage 1 (A) and Passage 5 (B) cultured in 3dGRO™ Spheroid Medium.
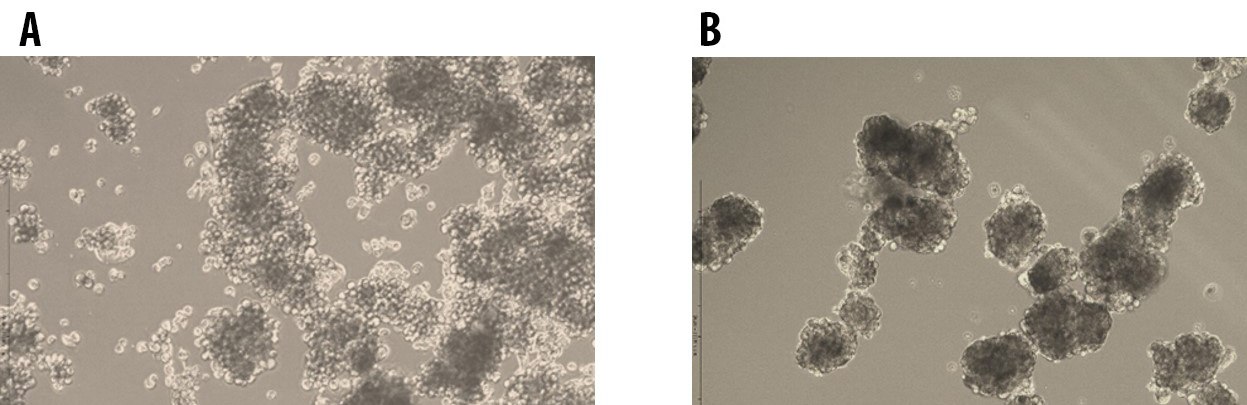
Figure 2.Tumorsphere formation of E006AA Prostate Cancer Cells at Passage 1 (A) and Passage 5 (B) cultured in 3dGRO™ Spheroid Medium.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?