Forskolin-Induced Swelling Assays using Millicell® Microwell Plates
Forskolin-Induced Swelling Assays in Cell Culture
In the realm of cystic fibrosis (CF) therapeutics, the pursuit of novel methodologies is paramount. One such approach involves the integration of the forskolin-induced swelling assay with organoid models, a technique that holds promise for elucidating CF pathophysiology and evaluating therapeutic interventions.
Organoids, intricate three-dimensional structures derived from either induced pluripotent stem cells (iPSCs) or from primary tissues, provide a biologically relevant platform for recapitulating CF-associated molecular and cellular phenomena. Central to this approach is the forskolin-induced swelling assay, which relies on the activation of the cystic fibrosis transmembrane conductance regulator (CFTR) by forskolin to evaluate chloride transport, a pivot indicator of CFTR functionality. Notably, the integration of Millicell® Microwell technology enhances the efficiency and reproducibility of organoid-based assays, enabling robust analysis of CFTR function in a high-throughput manner.
Here, we use our 600 µm Millicell® Microwell plate to cultivate similar-sized iPSC-derived colon organoids from single-cell suspensions monitored over a 5-day time-lapse period. On the fifth day post-organoid formation, we utilize the forskolin-induced swelling assay, observing over approximately 26 hours using time-lapse imaging to assess the functionality of the CFTR within these organoids.
Materials
- 600 µm Millicell Microwell plate (MC96U6)
- 3dGRO® iPSC Human Colon Organoid (SCC300)
- Corning® Matrigel® Basement Membrane Matrix (CLS354234)
- Phosphate Buffered Saline (PBS) (P2272)
- ROCK inhibitor (SCM075)
- TrypLE™ Express Enzyme
- Hemocytometer
- Millicell® DCI Digital Cell Imager (MDCI10000)
- Trypan Blue (T8154)
- 3dGRO® Human Colon Organoid Expansion Media (SCM304)
- DMSO (D8418)
- Forskolin (F3917)
- MuviCyte Instrument
Human iPSC Colon Organoid Growth Protocol
Pre-Seeding 3dGRO® Human Colon Organoid in Millicell Microwell Plate
- Remove a 600 µm Millicell Microwell plate from the fridge and sterilize the plate with its outer plastic wrapping with 70% alcohol.
- Place plate in the hood and remove the plastic wrap while keeping the plate level.
- Remove the lid and the sealing layer.
NOTE: Sealing layer can either be on the underside of the lid or on top of the opened place.
- Remove the shipping buffer in each well with an aspirating pipette from the pipetting port and the cell seeding chamber. Please refer to the User Guide for detailed instructions.
- Add 150 µL of culture media to the pipetting port and incubate plate in a 37°C incubator with 5% CO2 for a minimum of 30 minutes to rehydrate the hydrogel microwells.
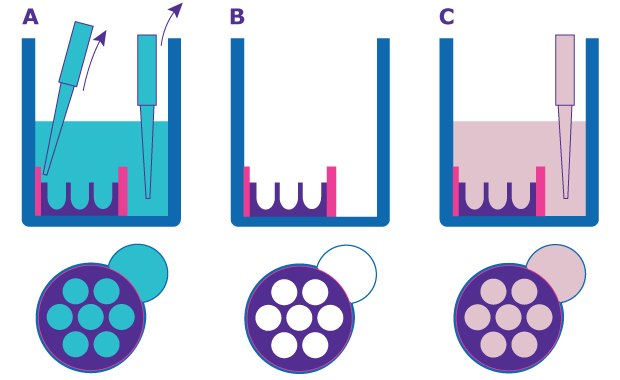
Figure 1.Schematic of Millicell® Microwell plate preparation. A. and B. Remove shipping buffer from the cell seeding chamber and pipetting port. C. Add 150 µL of growth medium to the pipetting port.
Preparing 3dGRO® Human Colon Organoid for seeding
This protocol is based on a 20 x 25 µL domes each with 80-90% occupancy.
- Remove a 6-well plate containing 20 x 25 µL Matrigel® dome of 3dGRO® Human Colon Organoid (SCC300) on day 7 of culture in a well from the incubator and place in the tissue culture hood.
- Aspirate media and add 1 mL of ice-cold 1X PBS + 10µM ROCK inhibitor to the well containing 20 x 25 µL Matrigel® domes.
NOTE: The 20 x 25 µL domes of 3dGRO® Human Colon Organoid are sufficient to seed 60 wells of the 96-well Millicell® Microwell plate @ 200 cells/µwell. It’s recommended to use at least 40 x 25 µL domes of 3dGRO® Human Colon Organoid at about 80-90% occupancy per domes for seeding at 200 cells/µwell for the whole plate.
- Dislodge Matrigel® domes with a P-1000 pipette by dispensing the 1 mL of 1X PBS + 10 µM ROCK inhibitor at the domes until all domes are dislodged and broken up.
- Transfer the organoid + Matrigel® suspension into a 15 mL conical tube.
NOTE: Use a maximum of 20 x 25 µL domes per 15 mL conical tube. If you have more than 20 x 25 µL domes, use additional 15 mL conical tube.
- Wash the residual Matrigel® domes from the well with 1 mL ice-cold 1X PBS + 10µM ROCK inhibitor and then transfer to the 15 mL conical tube with the other.
- Spin down at 1500 rpm for 10 minutes @ 4°C.
- Transfer tube to the tissue culture hood and carefully aspirate off the supernatant and the Matrigel® layer with an aspirating pipette connecting to a P-200 pipette tip.
- Wash pellet with 1 mL ice-cold 1X PBS + 10 µM ROCK Inhibitor by pipetting up and down 4 to 5 times.
- Spin down at 1500 rpm for 10 minutes at 4°C.
- Transfer the tube to the tissue culture hood and carefully aspirating off the supernatant and the Matrigel® layer with an aspirating pipette connecting to a P-200 pipette tip.
- Add 1 mL of TrypLE™ Express + 10µM ROCK Inhibitor to the pellet and pipet up and down vigorously 10 times. Place in a 37°C incubator with 5% CO2 for 45-60 minutes, pipetting every 5 minutes until suspension is mostly single cells.
- Take 10 µL sample after 20 minutes of incubation at 37°C and add to a hemocytometer.
- Observe cells under a microscope, such as the Millicell® DCI Digital Cell Imager, to make sure cells are mostly single cells.
NOTE: it’s crucial that cells are mostly single cells before seeding in order to get similar size organoids.
- Repeat this step every 5 minutes after 20 minutes until suspension is mostly single cells.
- When suspension is single cell dissociated, take 40 µL of the single cell suspension and add 40 µL of Trypan Blue.
- Add 4 mL of 3dGRO® Human Colon Organoid Expansion Media (SCM304) + 10 µM ROCK inhibitor to the rest and spin down at 1500 rpm for 5 minutes at 4°C.
- While spinning, add 10 µL of cells + Trypan Blue suspension to each side of the hemocytometer and count.
- Transfer the tube to the tissue culture hood and carefully aspirate off the supernatant with an aspirating pipette connecting to a P-200 pipette tip.
Seeding 3dGRO® Human Colon Organoid in a 600 µm Millicell® Microwell Plate
- Aspirate off supernatant from step 23 above and resuspend the cell pellet in 3dGRO® Human Colon Organoid Expansion Media + 10 µM ROCK Inhibitor for a cell density of 220,000 cells/mL.
NOTE: The 20 x 25 µL domes of 3dGRO® Human Colon Organoid is sufficient to seed 60 wells of the 96-well Millicell® Microwell Plate at 200 cells/µwell. It’s recommended to use at least 40 x 25 µL domes of 3dGRO® Human Colon Organoid at about 80+ percent occupancy for seeding at 200,000 cells/µwell for the whole 96 wells.
- Remove Millicell® Microwell plate from the 37°C incubator with 5% CO2 and aspirate off the media with an aspirating pipette from the pipetting port and the cell seeding chamber (Figure 2). Please refer to the User Guide for detailed instructions.
- Transfer cells to a sterile reservoir and use a 12-channel pipette to seed 50 µL of cells directly above the center of the cell seeding chamber of the 600 µm Millicell® Microwell plate for a 200 cells per µwell (Figure 2).
- Allow cells to sediment for 20-30 minutes in the 37°C incubator.
- While cells are incubating, prepare enough 3dGRO® Human Colon Organoid Expansion Media + 10 µM ROCK Inhibitor + 2.1% GFR Matrigel®.
NOTE: Make sure 3dGRO® Human Colon Organoid Expansion Media + ROCK inhibitor is ice-cold before adding GFR Matrigel® into it.
- Transfer plate to the tissue culture hood and add 150 µL of 3dGRO® Human Colon Organoid Expansion Media + 10 µM ROCK Inhibitor + 2.1% GFR Matrigel® very slowly to the pipetting ports of each well with a multi-channel pipette (Figure 2).
NOTE: Final percentage of GFR Matrigel® should be at 1.6%.
- Place the Millicell® Microwell plate in a 37°C incubator with 5% CO2 for culturing.
NOTE: Organoids will take 4-5 days to form.

Figure 2.Schematic of seeding single cell from 3dGRO® Human Colon Organoids into Millicell® Microwell plate. Aspirating media from both pipetting port and cell seeding chamber (Step 25). Seeding single cell suspension directly above the center of the cell seeding chamber (Step 26). Adding media very slowly to the well via the pipetting port (Step 29).

Figure 3.Still images from time-lapse of 3dGRO® Human Colon Organoids (SCC300) formation in 600 µm Millicell® Microwell plate over the course of 5 days. A. SCC300 at 200 cells per µwell immediately after seeding on day 0, B. SCC300 organoids formation on day 1, C. SCC300 organoids formation on day 3, D. SCC300 organoids formation on day 5.
(Please use the full-screen expand option to view.)
Figure 4. Time-lapse video of 3dGRO® Human Colon Organoids (SCC300) formation in 600 µm Millicell® Microwell Plate over the course of 109 hours. SCC300 seeded at 200 cells per µwell.
Forskolin-Induced Swelling Assay Protocol
Start Forskolin-Induced Swelling Assay (FIS) starting on day 5 post-seeding.
- Prepare enough 3dGRO® Human Colon Organoid Expansion Media + 10 µM ROCK Inhibitor + 1% GFR Matrigel® at 26.67 µM Forskolin and its vehicle (0.267% DMSO) before starting assay.
- Remove the 600 µm Millicell® Microwell plate containing the 3dGRO® Human Colon Organoids at Day 5 post-seeding from the 37°C incubator with 5% CO2 and place in a tissue culture hood.
- Remove 150 µL of media from each well via the pipetting port with a P-200 multi-channel pipette. Please refer to the User Guide for detailed instructions.
- Transfer the untreated, vehicle and the Forskolin samples into separate sterile reservoirs.
- Set up the FIS assay as follows:
- For Untreated Samples: add 150 µL of 3dGRO® Human Colon Organoid Expansion Media + 10µM ROCK Inhibitor + 1% GFR Matrigel® very slowly to the appropriate wells via the pipetting port.
- For Vehicle Samples: add 150 µL of 3dGRO® Human Colon Organoid Expansion Media + 10µM ROCK Inhibitor + 1% GFR Matrigel® + 0.267% DMSO very slowly to the appropriate wells via the pipetting port.
- For Forskolin Samples: add 150 µL of 3dGRO® Human Colon Organoid Expansion Media + 10µM ROCK Inhibitor + 1% GFR Matrigel® + 26.67 µM Forskolin very slowly to the appropriate wells via the pipetting port.
- Transfer the plate to the MuviCyte instrument in a 37°C incubator with 5 % CO2 to capture time-lapse video of the swelling for the desired duration. Here, the time-lapse video was captured for ~26 hours at 37 minutes interval for a total of 42 intervals (Figure 5).

Figure 5.Time-lapse of Forskolin Induced Swelling (FIS) assay on 3dGRO® Human Colon Organoids. Untreated control w/o Forskolin at baseline and 4.3 h (Well G7). Vehicle Control (0.2% DMSO) at baseline and 4.3 h (Well D8). Test Sample @ 20 µM Forskolin at baseline and 4.3 h (Well D7). Red circles in Well D7 show examples of organoids that swell at 4.3 h compared to those at baseline.
(Please use the full-screen expand option to view.)
Figure 6. Time-lapse video of Forskolin Induces Swelling assay on 3dGRO® Human Colon Organoids. Untreated control w/o Forskolin at baseline and 4.3 h (Well G7). Vehicle Control (0.2% DMSO) at baseline and 4.3 h (Well D8). Test Sample @ 20 µM Forskolin at baseline and 4.3 h (Well D7).
Tips and Tricks
- Make sure both HepG2 and 3dGRO® Human Colon Organoids are completely single cell dissociated into Millicell® Microwell plate. This will help to generating similar sized spheroids/organoids.
- When changing media, make sure to gently and slowly do so via the pipetting ports. Removing and adding media too fast will disrupt the spheroids/organoids in the µwells.
- For hu iPSC-derived Colon Organoids, make sure to add the 150 µL of the Expansion Media + 2.1% GFR Matrigel® + 10 µM of ROCKi at the end of cell seeding. Final GFR Matrigel® concentration after seeding should be 1.6%. Organoids in suspension need a very low percentage of GFR Matrigel® to grow. It’s recommended to start with a final concentration between 1-2% of GFR Matrigel®.
- For hu iPSC-derived Colon Organoids, always includes 10 µM of ROCKi + 1% GFR Matrigel® to the expansion media and change media every 2 days after seeding. ROCKi is required to keep single cell dissociated hu iPSC-derived colon organoids to grow and form organoids.
- For hu iPSC-derived Colon Organoids, it is recommended to use passage 15 or lower for generating organoids in Millicell® Microwell plate from single cell suspension.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?