Millicell® Microwell Plates Frequently Asked Questions (FAQs)
3D cell culture has emerged as an important tool for researchers studying cancer, developing novel drugs, and for other research applications. Millicell® Microwell 96-well plates support reliable and reproducible organoid culture with array of ultra-dense microwells in hydrogel. To learn more about Millicell® Microwell plates, visit our Knowledge Center.
What are the application areas where Millicell® Microwell plates have been used?
Millicell® Microwell 96-well plates have been used to grow various 3D cell culture models of healthy and tumor origin, including intestine, liver, and pancreas. This is including but not limited to colorectal organoids, mouse intestinal organoids, and blood-brain barrier spheroids (self-assembly of astrocytes, pericytes, and endothelial cells).
Learn more about Millicell® Microwell 3D cell culture applications.
Different spheroid types, including cancer line spheroids (HCT 116), primary hepatocyte spheroids, and bone marrow spheroids, have also formed on Millicell® Microwell plates.
Millicell® Microwell plates have been used in multiple cellular applications, including ADME-Tox assays, neuroscience, oncology, immunology, developmental biology, and cell therapies.
What are the recommended cell seeding densities for each microwell size for Millicell® Microwell plates?
We have tested multiple cell types on Millicell® Microwell plates. For adult-stem cell derived organoids, we recommend starting at 200 cells per microwell (μWell). For other cell lines or cell types, our scientists have recommended the following ranges of cell seeding densities per microcavity size:
- 400 μm: 100 - 2000 cells/ μWell (from proliferative to non-proliferative cells)
- 600 μm: 200 - 5000 cells/ μWell (from proliferative to non-proliferative cells)
Note: it’s best to start with the minimum recommended number of cells.
How should Millicell® Microwell plates be stored?
Millicell® Microwell 96-well plates are made of polystyrene and the hydrogel is made of poly-ethylene glycol. The PEG hydrogel has a molecular weight of 10.000 +/- 10% and a purity of >90%; in most cases the hydrogel and plate will not bind to small molecules. The plates should be stored upon receipt at 4˚C and are stable at 4˚C for 18 months from the date of manufacture.
The hydrogel will not dry out during the first 6 months of storage if the plate remains unopened but must remain hydrated in buffer to be stable. If the hydrogel dries out, it can no longer be used.
Are Millicell® Microwell plates automatable?
Millicell® Microwell 96-well plates are 127.90 x 85.60 x 14.45 mm (l x w x h), SLAS-standard, and are compatible with standard laboratory equipment, such as multichannel pipettes or high-content imagers. ANSI SLAS standards define the standards for microplate development in terms of dimensions and well positions. Millicell® Microwell 96-well plates therefore allow researchers to establish automated organoid workflows including hydrogel equilibration, cell seeding, medium exchange, and compound exposure with liquid handlers.
Because Millicell® Microwell plates are SLAS-compatible and generate a high volume of organoids per well in the same focal plane, they are well suited for screen programs. The plates allow multiple replicates per well, maximizing the datapoints that can be generated. Moreover, multiple organoid generation in a single well allows for multiple assays to be performed independently of their limit of detection.
What are the microwell sizes for Millicell® Microwell plates?
Millicell® Microwell plates are offered in two microwell size options:
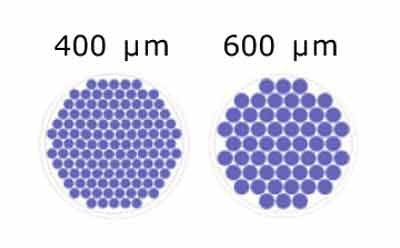
Figure 1.Schematic of microwell diameters in Millicell® Microwell 96-well plates.
Different diameters of microwells (400m, 600m) help accommodate the different types and sizes of organoids. If your organoids develop in a short time from a low number of cells or are small, the smaller microwell diameters would allow for higher microwell density per area. This would also result in more datapoints per well. Larger microwell sizes are suggested for organoids that grow for longer times or to larger sizes.
For each microwell size in Millicell® Microwell 96-well plates, the recommended well volumes are 50 μl seeding chamber and 150 μl for the pipetting port. The dead volume in the microwells depends on the diameter of the microwell, but it should be below 5 μl after buffer and media removal from the seeding chamber.
Can I use the unused wells in Millicell® Microwell 96-well plates?
Yes, you can use different wells of one plate for different experiments. However, it is important to make sure that the hydrogel in the unused microwells stay hydrated and that the sterility of the wells is maintained.
We do not recommend reusing the plate after washing, as we cannot guarantee the quality of the plate when reusing it.
Do I need to add extracellular matrix (ECM) to my organoid cultures on Millicell® Microwell plates?
ECM needs will depend on the organoid type. For organoids that are expanded and embedded in basement membrane extract (BME), it may be necessary to mix ECM with the culture media. For organoids that require Matrigel®, usually added at 1.5-2%, it will need to be added in every medium change.
Millicell® Microwell plates are compatible with a wide range of ECM gels, including collagen-I, Matrigel®, Matrigel® growth factor reduced BME, Cultrex BME, and laminin. The concentration of ECM will largely depend on the application, as different models require different concentrations of ECM. We recommend at least equal to or superior to the laminin concentration of Matrigel®, or approximately 150 μg/ml. ECM optimization will be required, as users should calculate from the Matrigel protein concentration their equivalent concentration in their alternative ECM and some organoids may require higher concentrations of ECM than others.
How do I prepare Millicell® Microwell plates for cell seeding?
The PEG coating on Millicell® Microwell plates is naturally cell-repellent, so no coating is needed before seeding the cells. Users should first aspirate the storage buffer from the pipetting port and the cell seeding chamber in the wells. Then add 150 μl of the media of interest into the pipetting port and incubate for 15 min in the cell incubator or 30 minutes at room temperature. Remove media from the pipetting port and cell seeding chamber and proceed with the cell seeding protocol.
You will need 50 μl of a single cell suspension per well in Millicell® Microwell plates. To evenly seed cells into the microwells, remove the media until the hydrogel microwell pattern appears. The cell density should be optimized for your cellular application and depends on the growth rate of the desired cells and final organoid size. Organoid size can be controlled by adjusting the starting cell seeding density.
If the seeding step is performed correctly, there should not be any cells in the pipetting port after seeding. If there are cells in the pipetting port, this could be from two potential causes: 1) the pipetting port wasn’t dried properly before cell seeding, or 2) the volume is over 50 μl. To prevent this effect, make sure the pipetting port is dry before cell seeding, and you use the correct seeding volume.
The seeding ring that surrounds the hydrogel separates the wells from the pipetting port. It also has a meniscus-breaking effect, enabling homogeneous cell seeding in the wells. Typical seeding uniformity in Millicell® Microwell plates is homogeneous, biological variations in the number of cells in each microwell within the same well are less than 5%.
Do I need to centrifuge Millicell® Microwell plates after cell seeding?
No, Millicell® Microwell plates do not need to be centrifuged after cell seeding. Millicell® Microwell technology enables cell seeding and aggregation in a single pipetting step. The seeding ring within the well separates the hydrogel from the pipetting port and enables homogeneous cell seeding within a well through its meniscus-breaking effect.
However, to prevent single cells from scattering at the bottom of the hydrogel after the cells have sedimented, a short centrifugation at a low speed, such as 100 x g, may help cell aggregation within the microcavities.
When there are too little cells or when they are not very motile, some cell types do not aggregate in the center of the microwells. Users can avoid cells forming multiple organoids or spheroids in the microwells by increasing the seeding density.
How can I avoid damaging the hydrogel in Millicell® Microwell plates?
The PEG hydrogel is sensitive, so if the hydrogel is disturbed by the pipette tip it may damage the microwells and risk organoid or microtissue loss. To avoid damaging the hydrogel, always be sure to use the pipetting port for media removal and loading. When performing a cell seeding protocol, cells should be added directly above the cell seeding chamber.
How do you aspirate the media from the inner seeding ring without damaging the hydrogel?
Remove the media from the pipetting port using an aspirator and a Pasteur pipette (Figure 2). Once the media is removed, carefully access the cell seeding chamber by sliding the pipette tip on the side of the well until you feel the resistance of the seeding ring. Aspirate the remaining buffer from there without touching the hydrogel until the microwell arrays become visible. Full buffer or media removal is not necessary.

Figure 2.Millicell® Microwell 96-well plate pipetting port for automated and non-automated liquid handling without organoid loss.
How often should I change the medium?
The frequency of media change depends on the cell type and specific application protocols. Media should be changed every 2-3 days, or as frequently as needed. You can also safely sample media out through the pipetting port for downstream assays, such as mass spectrometry.
How long do the organoids take to form using Millicell® Microwell plates?
Organoid formation time strongly depends on the type of cells used, their rate of proliferation, and the cell seeding density that is used. These parameters will need to be optimized depending on your applications. It can take 2-5 days for the cells to fully compact. The resulting organoids are homogeneously distributed in size, with variations from biology. The organoids and spheroids cultured in Millicell® Microwell plates can be cultured for as long as desired.
Can I establish co-cultures with multiple cell types using Millicell® Microwell plates?
Yes, you can establish co-cultures using Millicell® Microwell plates several ways. You can aggregate multiple cells by mixing them before cell seeding, such as with our blood-brain barrier model, or you could add a second cell type for co-culture with the organoids once they have formed, such as with immune cells as seen in our T-cell killing assay protocol.
How can I harvest organoids from Millicell® Microwell plates?
Single organoids can be easily harvested from the microwells because the cells grow in an open and solid matrix-free environment. Organoids or spheroids that contain some of the hydrogel can also be harvested; PEG is cell-inert and can be removed after organoid centrifugation.
Organoid harvesting involves a simple pipetting step without cumbersome extraction protocols (Figure 3). Users should gently pipette 150 μl up and down in the cell seeding chamber 4-5 times to resuspend the organoids in the medium. The organoids can then be harvested into a tube. To ensure all organoids are recovered from the microwells, an additional washing step may be required.
The organoids can stick to pipette tips and tubes. To avoid this, we would recommend that users pre-coat tips and tubes with 2% BSA in PBS or media. Pipette tips should be coated by pipetting the solution up and down several times before collecting the organoids, and tubes should be allowed to incubate with the coating solution for at least 15 minutes on ice.
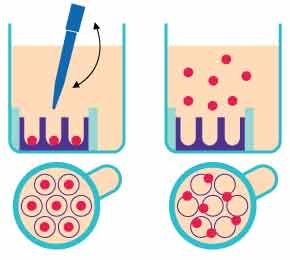
Figure 3.Organoid harvesting using Millicell® Microwell plates.
Once organoids have formed, how do I proceed to assay?
Once organoids form, you can directly use them in your application or assay of choice. Organoids formed on Millicell® Microwell 96-well plates can be immunostained and imaged directly on the plate, the media or supernatant can be used in downstream cellular assays, and the plates can be used for organoid screening.
All the steps of immunofluorescence can be performed on Millicell® Microwell plates from fixation, permeabilization, blocking, antibody incubation, and imaging. Millicell® Microwell 96-well plates are compatible with transmitted light and epifluorescence imaging. The organoids form in a single focal plane, which allows users to image multiple organoids at the same time. This establishes an efficient image-based workflow, minimizing the acquisition time and maximizing extracted data.
Immunostaining Protocol1:
- PFA 4% FIXATION (30 min, RT) - use pipetting port
- 3x PBS washes (5 min, RT) – use pipetting port, 150 μl at a time
- Permeabilization with 0.2% Triton X-100 in PBS (30 min, RT) – add 150 μl in the pipetting port
- 3x PBS washes (5 min, RT) - use pipetting port, 150 μl at a time
- Blocking with 10% serum (species of secondary antibody) in 0.02% Triton X-100 in PBS (30 min, RT) - use pipetting port, 150 μl at a time
- Blocking buffer removal from pipetting port and from seeding chamber leaving as little liquid as possible, but without disturbing the samples
- Addition of primary antibody desired in blocking buffer (see step 5) – 50 μl on top of organoids, drop by drop (ON, 4°C)
- 3x PBS washes (5 min, RT) – use pipetting port, 150 μl at a time
- Addition of secondary antibody desired in blocking buffer (see step 5) – 50 μl on top of organoids, drop by drop (ON, 4°C)
- 3x PBS washes (5 min, RT at least, up to 4 hours) – use pipetting port, 150 μl at a time
What are the fluorescent imaging limitations of the plastic bottom Millicell® Microwell plates?
Plastic bottom Millicell® Microwell plates are suitable for fluorescence imaging at low magnification, such as 4X or 10X. Because the organoids or spheroids form in a single focal plane, users can image the microtissues in a few z-stacks. For high magnification, the organoids can be harvested after staining and transferred to a glass slide for imaging.
Are Millicell® Microwell plates compatible with fluorescent or colorimetric (O.D.) readings and assays?
We offer clear, plastic bottom Millicell® Microwell plates that are compatible with several cell viability assays, including fluorescence image-based assays, such as the LIVE/DEAD™ Kit, or luminescence-based assays, such as the CellTieter-Glo® 3D assay. Fluorescence and colorimetric assays can be performed on the plates, but because they are transparent, we recommend sampling out in a white- or black-well assay plate for optimal assay results.
The PEG hydrogel does not interfere with CellTiter-Glo® 3D or other luminescence-based assays, as it is a cell-inert compound. CellTiter-Glo® 3D reagents contain a lytic component that lyses cells and a compound that reacts to the presence of ATP from the lysed cells, neither of which interact with PEG.
Can I extract RNA/DNA/protein from my 3D cultures on Millicell® Microwell plates?
Yes, you can extract RNA, DNA, and protein from 3D cultures grown on Millicell® Microwell plates. After harvesting the organoids, you can proceed with your in-house protocol or centrifuge the organoids and proceed with the RLT buffer from Qiagen according to their protocol. Depending on the amount of microtissue and the size of the organoids, you may have to pool several wells to have enough starting material for extraction protocols.
Can I cryosection my organoid samples from Millicell® Microwell plates?
Yes, microtissue samples in the microwells can be fixed and cryopreserved by successive incubations in sucrose gradients. The hydrogel that contains the organoids can then be scooped out, embedded in OCT, snap-frozen, and cryosectioned. See above for immunostaining protocol.
Cryosectioning protocol2:
- PFA 4% FIXATION (15-30 min, RT) - use pipetting port
- 3x PBS washes (5 min, RT)
- Incubation 10% sucrose (ON, 4°C - use pipetting port to add the solution, correct for 50 ul remaining in pipetting port)
- 2X PBS washes
- Incubation 20% sucrose (ON, 4°C - use pipetting port to add the solution, correct for 50 ul remaining in pipetting port)
- 2X PBS washes
- Incubation 30% sucrose (ON, 4°C - use pipetting port to add the solution, correct for 50 ul remaining in pipetting port)
- Scoop the hydrogel with spheroids out of Millicell® Microwell plates
- Embedding in OCT using a cassette - keep at 4°C ON
- Snap-freeze sample
- Proceed to cryosection and antibody incubations according to your in-house protocols.
References
To continue reading please sign in or create an account.
Don't Have An Account?