Tuning the Elastic Moduli of Corning® Matrigel® and Collagen I 3D Matrices by Varying the Protein Concentration
A complex set of biophysical (e.g., topography, stiffness, viscosity, porosity) and biochemical (e.g., nutrients, matrix composition, cell-matrix interactions) cues control cell biology1. In order to mimic the in vivo cellular behavior for understanding basic biology, tissue engineering, and regenerative medicine applications, it is imperative to better elucidate and control these interactions. The importance of matrix elasticity is being increasingly studied and the stiffness (or elastic modulus) of naturally derived as well as engineered hydrogel substrates has been shown to direct stem cell differentiation2, effect cell migration3, modulate force of muscle contraction4, and influence cell spreading and adhesion5. Interestingly, stiffness of tissues in abnormal conditions can also vary: normal rat liver tissue (0.3 to 0.6 kPa) compared to cirrhotic liver (3 to 12 kPa)6,7, and normal breast tissue (1.2 kPa) compared to breast tumors (2.4 to 4.8 kPa)8. This phenomenon may be causative and not necessarily only an outcome of a disease state9.
Section Overview
Rigidity in Cell Culture
Lineage specific differentiation of human mesenchymal stem cells (hMSCs) has been shown to vary with matrix stiffness, where hMSCs were directed into particular phenotypes: neurons (matrix stiffness of 0.1 to 1 kPa), myoblasts (8 to 17 kPa), and osteoblasts (25 to 40 kPa)2. As rigidity of the cross-linked polyacrylamide gels increased, cells increased their secretory response (e.g., Collagen I). After several weeks of culture and based on matrix rigidity, the specific lineage of the cells was committed. In another study, liver cell differentiation into abnormal phenotypes (myofibroblasts) was driven both by soluble factors and increasing matrix stiffness6,7. Following this change, an increased amount of abnormal extracellular matrix (ECM) was produced, resulting in fibrosis (scarring).
Human prostate carcinoma tumor cell (DU-145 cell) migration speed has been influenced by increasing Corning® Matrigel® matrix stiffness, and the maximum speed was observed at intermediate stiffness3. Other factors found to affect migration speed included cell adhesion and proteolytic activity of the cells. Additionally, pore size within the matrix decreased as Matrigel® matrix concentration increased; however, this only played a minor role in migration speed.
Increasing Matrigel® matrix concentration (from 10% to 40%) was also demonstrated to increase the contraction force of engineered skeletal muscle constructs4. Cell survival and function were affected minimally by matrix composition.
Extracellular Matrices
Basement membrane is a thin layer of ECM proteins underlying all tissues in vivo10. Corning® Matrigel® matrix is a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm.
(EHS) mouse sarcoma, a tumor rich in extracellular matrix proteins, including Laminin (a major component), Collagen IV, heparan sulfate proteoglycans, entactin/nidogen, and a number of growth factors that are found in normal EHS tumors11,12. Matrigel® matrix has been used in a wide range of applications such as stem cell culture, cell attachment and differentiation, angiogenesis assays, tumor growth, and tissue engineering13,14.
Collagen is the most abundant protein present in animals, providing structural and mechanical support15,16. There are many different types of Collagen (about 28 known to date)15 and it is most plentiful in dermis, tendon, and bones14. The type I molecule of Collagen is a heterotrimer of 300 nm length and is composed of two alpha1(I) chains and one alpha2(I) chain.
Corning® Collagen I rat tail is prepared from rat tail tendons by acid extraction17. No enzymes are required for digestion, thus the telopeptides are preserved. Collagen can be used in a thin layer to promote cell attachment, study of tumor cell invasion and migration, fibrillogenesis studies, culture and differentiation of monocytes or macrophages, and in the maintenance of hepatocyte function18. Collagen I can also be used in 3D gel form or electrospun into membranes to support 3D cell growth and differentiation14.
Mechanical Properties of ECMs
Mechanical properties such as stiffness or elastic modulus of materials can be measured using a variety of techniques such as bulk compressive/tensile testing, rheometry, dynamic light scattering, atomic force microscopy (AFM), and nanoindentation19,20.
In rotational or dynamic rheometry, viscoelastic material properties are measured by applying oscillatory shear strain on the sample, most commonly performed in a parallel-plate set-up. For example, in one study, different Corning® Matrigel® matrix (standard product) concentrations ranging from 50% to 100% were tested using a parallel-plate rheometer, and the stiffness (G') linearly increased from approximately 10 to 50 Pa22. In a different report, storage modulus measured using a stress-controlled rheometer for Matrigel® matrix (high concentration product) concentrations of 4.4, 8, and 17 mg/mL was approximately 20, 70, and 300 Pa, respectively21. A stiffness value of 44 Pa has been reported for Matrigel® matrix (high concentration product)22. Similarly, several publications have reported the stiffness values for Collagen I gels. Using a rotational rheometer, stiffness of Collagen I (rat tail, 2 mg/mL) was reported to be 9 Pa23. Furthermore, when Collagen I was mixed with Matrigel® matrix at a concentration of either 2 or 4 mg/mL, the stiffness increased to 13.4 and 40.7 Pa, respectively. In another report, the stiffness of Collagen I gels (rat tail and porcine) in the concentration range of 0.5 to 4 mg/mL increased from 0.5 to 100 Pa24.
Different mechanical responses are also observed when compressive forces are used instead of shear forces. Using displacement control unconfined compressive testing methodology, a modulus of 6 kPa was achieved for Collagen I (2 mg/mL) samples25. The constructs became increasingly stiffer with the addition of Matrigel® matrix (10% to 50% addition) with moduli ranging up to 10 kPa. The values obtained in this study are orders of magnitude higher than those from rotational rheometry studies.
Localized mechanical properties can be measured using AFM indentation by pressing a sharp tip into the sample. Under physiological conditions (37°C and aqueous), the average elastic modulus of Matrigel® matrix (standard product) measured using AFM with a micron-sized spherical tip was 443 Pa26. The differences in the stiffness results between rheological and AFM measurements could be due to differences in length scales (bulk vs. surface) in the two methods.
It should be noted that most of the cell culture work is typically performed on 2D substrates made of rigid materials such as glass and polystyrene, sometimes coated with a thin layer of ECMs. For example, polystyrene and glass substrates have a stiffness value in the GPa range which is orders of magnitude higher than that for ECM proteins (Pa to kPa range) causing the cells to show a non-natural behavior1. At the same time, results from measurements of mechanical properties of the thin ECM layers might be confounded by the bulk substrate stiffness.
In this paper, we examined the elastic moduli of the Matrigel® matrix and Collagen I gels as a function of the protein concentration. The rheological measurements were performed using single frequency temperature sweeps on a rotational rheometer.
Materials and Methods
Corning® Matrigel® Matrix Preparation
Corning® Matrigel® matrix, Growth Factor Reduced (Matrigel® GFR, Corning® Product No. 354230) and Corning® Matrigel® matrix High Concentration, Growth Factor Reduced (Matrigel® HC GFR, Corning® Product No. 354263) were thawed overnight in ice as directed in the Guidelines for Use27-29. The Matrigel® matrix products were diluted to the desired protein concentrations using the lot-specific protein concentrations provided on the certificates of analysis using ice-cold Dulbecco’s Modified Eagle Medium (DMEM). The contents of the vials were mixed by pipetting up and down and the calculated amount of Matrigel® matrix was pipetted using a positive displacement pipet, to ensure accuracy, and added to ice-cold DMEM in pre-chilled glass tubes held in ice. The diluted samples were vortexed gently and held in ice. As polymerization of Matrigel® matrix is temperature-dependent and non-reversible, it is important to prepare and hold the samples in ice. The diluted Matrigel® matrix samples were mixed by inversion immediately before loading the test samples onto the rheometer using a positive displacement pipet (0.680 mL). Each protein concentration was measured three times.
Samples with concentrations of 3, 6, 9, and 15 mg/mL were made using Matrigel® HC GFR. A sample with a concentration of 9 mg/mL was made using Matrigel® GFR. The volumes of Matrigel® matrix and ice-cold DMEM diluent required were calculated as follows:
| Desired final volume (mL) x Desired protein concentration (mg/mL) | = volume of Matrigel® matrix required (mL) |
| Lot-specific protein concentration (mg/mL) |
Desired final volume (mL) - volume of Matrigel® matrix required (mL) = volume of ice-cold diluent required (mL)
For example, to prepare a 10 mL solution at 9 mg/mL using Corning® Matrigel® matrix HC GFR released with a protein concentration of 19.1 mg/mL:
| 10 mL x 9 mg/mL | = 4.7 mL |
| 19.1 mg/mL |
For example, to prepare a 5 mL solution at 9 mg/mL using Matrigel® GFR released with a protein concentration of 9.5 mg/mL:
| 5 mL x 9 mg/mL | = 4.74 mL |
| 9.5 mg/mL |
Collagen I Preparation
Corning® Collagen I High Concentration, rat tail (Collagen HC rat tail, Corning® Product No. 354249) gelation is pH- and temperature-dependent, and the product must be neutralized in order to polymerize. Collagen HC rat tail was diluted using the alternate gelation procedure included in the lot-specific Certificate of Analysis30,31. All dilutions were prepared on ice in pre-chilled glass tubes.
To prepare the desired volume and protein concentration of Collagen I solutions, 0.1X the final volume of 10X Dulbecco’s Phosphate Buffered Saline (10X DPBS, Thermo Fisher), 0.023 mL of 1N Sodium Hydroxide (JT Baker) per mL of Collagen I were added and the final volume was adjusted using ice-cold DI-water. The contents of the tube were mixed by vortexing and the required volume of Collagen I was added to the solution using a positive displacement pipet, mixed by vortexing gently and held on ice. All diluted samples were mixed by inversion immediately before loading the test samples onto the rheometer using a positive displacement pipet (0.680 mL). Each protein concentration was measured in triplicate.
Samples with concentrations of 1, 3, and 7 mg/mL were made using Collagen HC rat tail.
For example, to prepare a 6 mL solution with a final Collagen I concentration of 7 mg/mL using Collagen HC rat tail (Lot No. 7016285) with a protein concentration of 9.82 mg/mL:
0.6 mL of 10X DPBS, 0.0984 mL of 1N NaOH and 1.02 mL of ice-cold DI-water were added to a pre-chilled glass tube and mixed by vortexing. Using a positive displacement pipet, 4.28 mL of Collagen HC rat tail was added to the glass tube.
Rheological Measurements
The samples were analyzed using the Kinexus® Ultra+ Rotational Rheometer (Malvern Instruments) using a parallel plate (40 mm roughened) configuration. Using a positive displacement pipet, samples (0.680 mL) were directly added to the center of the lower plate at a temperature of 5°C. The top plate was immediately lowered before the gel started to form, to a working gap of 0.5 mm. The temperature was ramped from 5°C to 37°C at a rate of 10°C/minute and the temperature was held at 37°C. A solvent trap was used to cover the sample and prevent any evaporation effects around the perimeter. Viscoelastic measurements were performed periodically at a single frequency of 0.5 Hz and 0.2% strain. Measurements were recorded until the elastic modulus reached equilibrium. The data was acquired and analyzed using the rSpace® software (Malvern Instruments).
Tips for Successful Usage of Corning® Matrigel® Matrix
Storage
- Store in a -20°C non-frost-free freezer.
- Minimize temperature fluctuations, do not store on the door or in a freezer that is opened frequently.
Thawing
- Pack in ice (completely submerge).
- Cover ice bucket and place in 2°C to 8°C location (cold room or refrigerator) overnight.
- Avoid repeated freeze thaw cycles.
- Thaw one-time use aliquots using the same guidelines, time may be adjusted based on the volume of the aliquot.
Diluting
- Use lot-specific protein concentration provided on the Certificate of Analysis to prepare dilutions.
- Keep Matrigel® matrix and all solutions on ice.
- Add Matrigel® matrix to ice-cold solutions (do not dilute with water, aggregates may form).
- Use pre-chilled tips, tubes, and cultureware.
- Use positive displacement pipets for accurate pipetting (due to viscosity).
Handling
- Plan ahead.
- Thaw Matrigel® matrix appropriately.
- Make one-time use aliquots.
- Prepare Matrigel® matrix aliquots immediately after thawing the vial.
- Store one-time use aliquots using the same recommended storage conditions.
- Aliquot non-diluted Matrigel® matrix only.
- Matrigel® matrix is temperature-sensitive and will start to gel above 10°C.
- Polymerization is non-reversible.
Tips for Successful Usage of Corning® Collagen I, Rat Tail
Storage
- Store at 2°C to 8°C.
- Do not freeze the product.
Diluting
- Use lot-specific protein concentration provided on the Certificate of Analysis to prepare dilutions.
- Use ice-cold solutions.
- Keep product and diluted samples on ice.
- Use positive displacement pipets for accurate pipetting (due to viscosity).
Handling
- Polymerization is pH- and temperature-dependent.
- Collagen must be neutralized in order to form a gel.
Results and Discussion
Proteins present in naturally derived ECMs such as Corning® Matrigel® matrix and Collagen I self-assemble into complex 3D fibrous structures32. Mechanical properties of these matrices regulate a myriad of cellular functions including cell spreading, proliferation, migration, and differentiation11. Mechanical properties such as stiffness or elastic modulus of materials can be measured using a variety of techniques such as bulk compressive/tensile testing, rheometry, AFM, and nanoindentation.
To investigate whether varying the protein concentration alters the elastic modulus of the Matrigel® matrix gels, Corning® Matrigel® High Concentration, Growth Factor Reduced (Matrigel® HC GFR; at a concentration of 19.1 mg/mL) was diluted using ice-cold DMEM and liquid samples were directly added to the rotational rheometer. The temperature of the bottom plate (Peltier-controlled) was ramped from 5°C to 37°C and then held at 37°C, which is the recommended gelation temperature for Matrigel® matrix.
The evolution of the elastic component (G') and the viscous component (G") of the complex shear modulus were observed over time and the sample was allowed to reach a state of equilibrium. Over a range of concentrations from 3 to 19.1 mg/mL for Matrigel® HC GFR, the elastic component of the shear modulus increased from 9.1 ± 0.3 Pa to 288.2 ± 9 Pa (Figures 1A and 1B). At the equilibrium state, the plateau value of the elastic component was larger than the viscous component indicating that the gel finally formed was elastic in nature (Figure 1C).

Figure 1.Increase of elastic modulus as a function of Corning® Matrigel® HC GFR concentration. (A) A plot of the elastic modulus (G') as a function of time for samples of Matrigel® matrix at different concentrations. Temperature was ramped from 5°C to 37°C and held at 37°C. (B) Plateau values of G' (in Pa) shown as a function of Matrigel® matrix concentration (n = 3). (C) Representative time course of elastic modulus and viscous modulus for Matrigel® matrix at a concentration of 15 mg/mL.
Corning® Matrigel® matrix is available as Matrigel® HC GFR (a high-concentration product) with a concentration range of 18 to 22 mg/mL and Corning® Matrigel® Growth Factor Reduced (Matrigel® GFR) with a concentration range of 7 to 10 mg/mL. The shear moduli (elastic component) of gels formed using the Matrigel® HC GFR and Matrigel® GFR at the same protein concentration (9 mg/mL) were measured and found to be comparable (Figure 2) indicating that mechanical properties of Matrigel® matrix are not influenced by the small differences in the processing of the high concentration and standard concentration products.
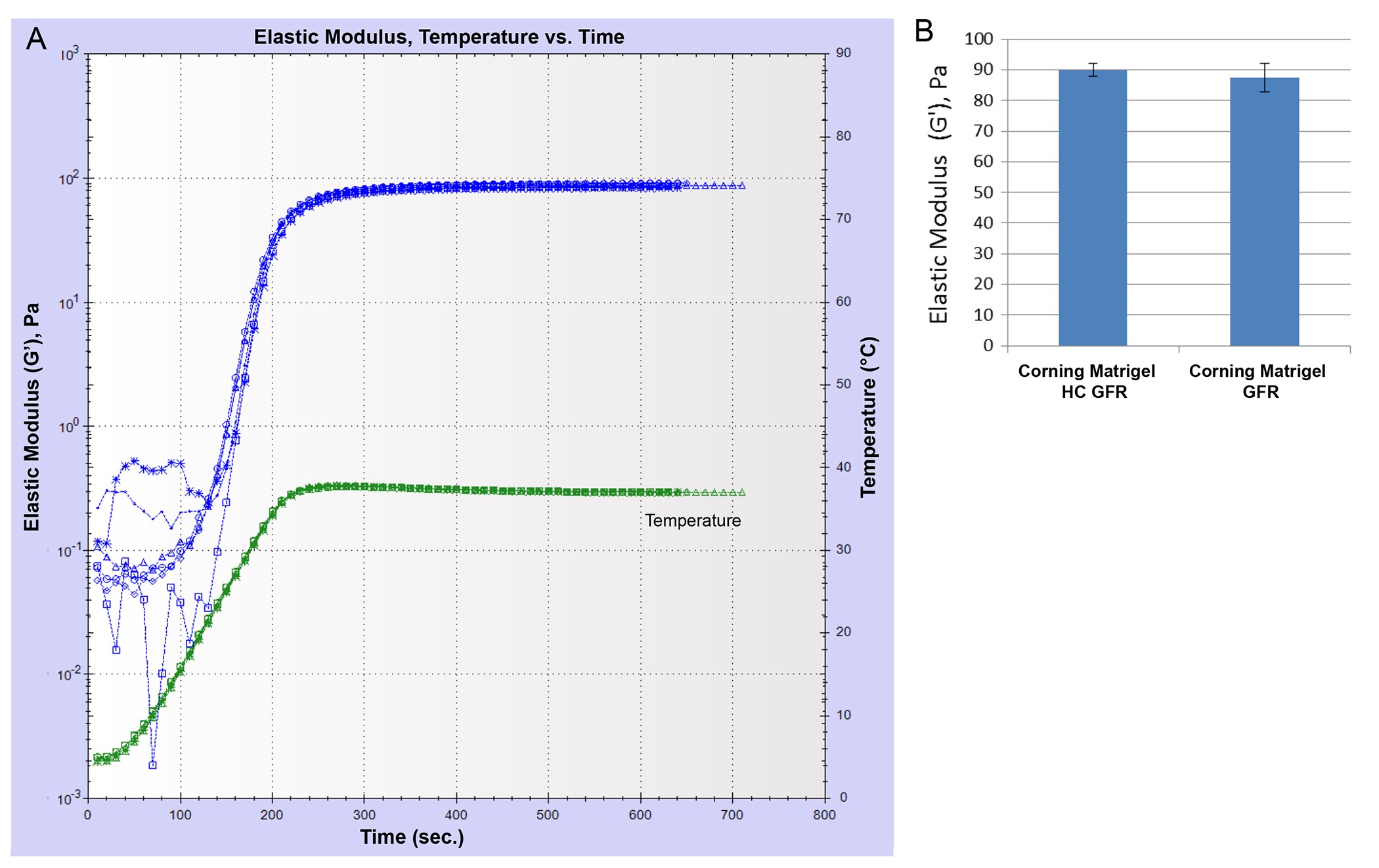
Figure 2.Comparison of elastic modulus (G') for two different Corning® Matrigel® matrix products formed at the same protein concentration (9 mg/mL). (A) A plot of the elastic modulus as a function of time for samples of Matrigel® HC GFR and Matrigel® GFR. Temperature was ramped from 5°C to 37°C and held at 37°C. (B) Plateau values of G' (in Pa) shown for the two different Matrigel® matrix products (n = 3).
To investigate whether varying the protein concentration alters the elastic modulus of the Collagen I gels, samples of different concentrations were created by neutralizing Corning® Collagen I High Concentration, rat tail (Collagen HC rat tail; at a concentration of 9.82 mg/mL) with 1 N NaOH solution. For Collagen HC rat tail concentrations of 1, 3, and 7 mg/mL, G' plateaued at 5.0 ± 0.6 Pa, 55.4 ± 11.0 Pa, and 341.8 ± 32.4 Pa, respectively (Figures 3A and 3B). Collagen I gels demonstrated an elastic behavior similar to Matrigel® matrix gels as seen when the plateau values of G' and G" were compared (Figure 3C).
Other factors that also play an important role in the gel formation and thereby affect the modulus are gelation temperature, temperature ramp, changes in general handling of the protein solutions, and more specifically for Collagen I pH of the solution. Rheometer parameters such as applied shear, frequency, working gap, and also the type of instrument used for the measurement could affect the final modulus values attained.
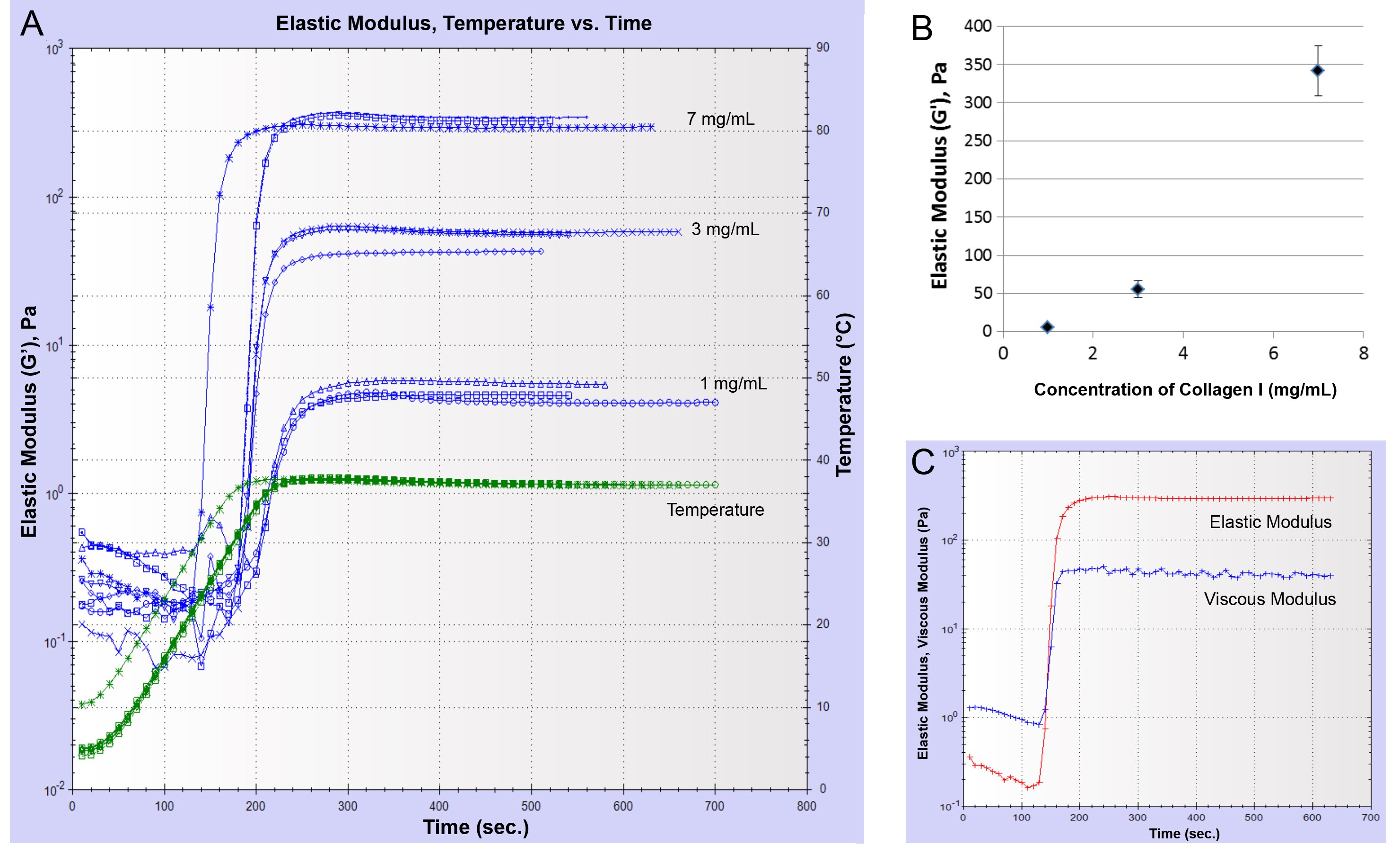
Figure 3.Increase of elastic modulus as a function of Collagen HC rat tail concentration. (A) A plot of the elastic modulus (G') over time for samples of Collagen I at different concentrations. Temperature was ramped from 5°C to 37°C and held at 37°C. (B) Plateau values of the shear modulus (elastic component, G') (Pa) vs. Collagen I concentration (n = 3). (C) Representative time course of elastic modulus and viscous modulus for Collagen I at a concentration of 7 mg/mL.
Conclusions
Cellular behavior is governed by a complex interplay of various physico-chemical factors. For instance, the effects of mechanical properties of substrates on cellular function with respect to understanding cell biology, and in applications of cell therapy and regenerative medicine continue to be an area of intense study. Numerous highly sensitive tools and techniques are available for the measurement of the mechanical properties of matrices. In this paper, we investigated the effect of protein concentration on the elastic moduli of Corning® Matrigel® matrix and Collagen I gels using a rotational rheometer. It was observed that the elastic modulus of both materials increased with increasing protein concentration and the gels were elastic in nature.
Acknowledgments
We are grateful to Dr. Philip Rolfe and Maria Lightfoot (Malvern Instruments, Westborough, MA) for giving us access to the Kinexus® Ultra+ Rheometer and assisting with set-up, data collection, and analysis.
Related Products
References
1-20
References
21-32
To continue reading please sign in or create an account.
Don't Have An Account?