Corning® Spheroid Microplates
Spheroid Formation Protocol
In vitro 3D cell culture models are widely recognized as more physiologically relevant systems compared to 2D formats. To recapitulate features of native tumor microenvironments, cancer cells can be cultured in Corning spheroid microplates, which combine the Corning Ultra-Low Attachment surface with innovative well geometry to provide an ideal tool for generating, culturing, and assaying 3Dmulticellular spheroids in the same plate, without the need for a transfer step.
This protocol describes a basic method for generating and culturing tumor spheroids in a 96-well spheroid microplate format. This basic protocol for culture and assay can be adapted for all spheroid microplates. Table 2 provides suggested volumes for miniaturization of the 96-well culture volumes to both 384- and 1536-well formats. Since plating volumes and seeding densities may vary with cell type and downstream application, assay specific optimization of conditions is recommended.
Methods and Materials
T-29 human colon cancer cells cultured in McCoy's 5a medium, A549 human lung cancer cells cultured in F-12K (Kaighn's Mod.) medium, and MCF7 human breast cancer cells.
HTB-22™ and DU 145 human prostate cancer cells both cultured in Dulbecco's Modification of Eagle's Medium (DMEM) were used for these studies. All growth media contained 10% Fetal Bovine Serum. Cell cultures were maintained according to ATCC recommendations and harvested using standard cell culture methods.
Initial plating densities for spheroid formation depend on factors such as cell type, duration of growth phase in a spheroidal format, and the desired size of spheroid at the time of assessment. To best evaluate spheroid formation and growth, cells were plated at densities of 40, 200, 1,000, 5,000, and 10,000 cells in 100 µL of growth media per well using 96-well spheroid microplates. Spheroid cultures were analyzed at 0, 24, 48, and 72 hours using CellTiter-Glo® 3D cell viability assay. The same seeding methods were used for all four cell lines.
- Harvest cells ensuring a healthy, single cell suspension.
Note: Cells can be passed through a 40 µM cell strainer or a 5 mL round bottom polystyrene test tube with cell strainer snap cap to achieve a single cell suspension.
- Prepare 5 mL dilutions for each seeding density (Table 1) in order to seed eight wells per seeding density for each time point (four 96-well spheroid microplates).
- Dispense 100 µL of cell suspension to eight wells per concentration. For control wells, dispense 100 µL of growth medium without cells.
Note: Spheroid microplates can be seeded manually* using a multichannel pipettor, or automated** using a liquid dispenser instrument. Manual liquid addition is adequate for both 96- and 384-well spheroid microplates. Automated liquid handling is recommended for a 1536-well spheroid microplate.
*During manual seeding, ensure pipet tips do not scratch the bottom or sides of the wells to avoid damaging the Corning® Ultra-Low Attachment surface coating.
**If using an automated liquid dispenser, increase the dilution volume to take into account priming the instrument and the length of tubing (10 mL).
- Place spheroid microplates in a humidified incubator set to 37 °C and 5% CO2.
- Evaluate spheroid formation and growth at 0, 24, 48, and 72 hour time points visually and performing a cell viability assay.
Visualization
Spheroid formation and growth was assessed via microscopic examination using an inverted microscope and by photographing spheroids for all five seeding densities at each time point.
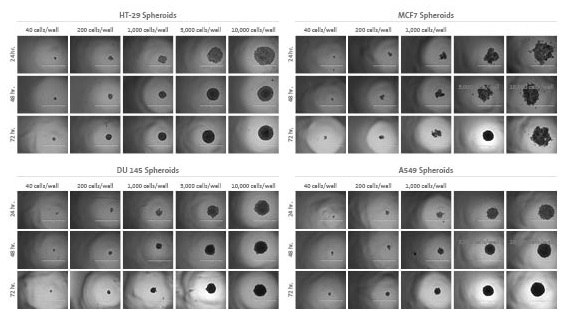
Figure 1.Spheroid formation and growth
Visual Observations:
At the 24-hour time point, cells formed loosely aggregated multicellular spheroids. After 48 hours of incubation, all but the MCF7 cells formed tight, defined spheroids. At 72 hours, HT-29 and A549 spheroids showed a slight increase in size when seeded at the lower densities (<5,000 cells/well), but DU 145 and MCF7 spheroids did not appear to increase in size after 48 hours.
Cell Viability Assay
The CellTiter-Glo® 3D Cell Viability Assay protocol was followed. Briefly, the CellTiter-Glo® 3D reagent was thawed; the reagent and assay plates were allowed to reach room temperature for 30 minutes.
The CellTiter-Glo® 3D reagent was added directly into wells in a 1:1 dilution (Table 2). The solutions were well mixed by shaking the plate for 5 minutes using an orbital plate shaker and then incubated at room temperature for a total of 30 minutes. After incubation, luminescent signal was read using an EnVision™ Multilabel Plate Reader (PerkinElmer Cat. No. 2104-0010).
Note: For the 0-hour time point (cell in suspension), plates were shaken for 2 minutes and incubated for a total of 10 minutes prior to reading signal.
Cell Viability Assay:
Exhibited continuous growth of HT-29 and A549 spheroids.
Growth inhibition of MCF7 and DU 145 spheroids observed.
Large spheroids (5,000 and 10,000 initial seeding densities) did not appear to have the linearity of growth exhibited with the smaller sized spheroids.
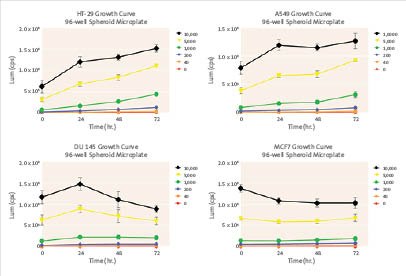
Figure 2.Cell Viability Assay
Materials
For more specific information on claims, visit the Certificates page at www.corning.com/lifesciences.
Warranty/Disclaimer: Unless otherwise specified, all products are for research use only. Not intended for use in diagnostic or therapeutic procedures. Not for use in humans. Corning Life Sciences makes no claims regarding the performance of these products for clinical or diagnostic applications.
For additional product or technical information, visit www.corning.com/lifesciences or call 800.492.1110. Outside the United States, call +1.978.442.2200 or contact your local Corning sales office.
To continue reading please sign in or create an account.
Don't Have An Account?