Materials for Dye-Sensitized Solar Cells
Introduction
While dye sensitization as the basis for color photography has been accepted for a very long time,1 attempts to use this principle for the conversion of solar light to electricity generally had resulted only in very low photocurrents, below 100 nA/cm2.2 In the mid-1980s, chemisorption of ruthenium-based dyes on high surface area titanium dioxide (titania or TiO2) through carboxylate bonds resulted in unprecedented photocurrents in the mA/cm2 range and incident photon-to-current conversion efficiency [IPCE, also called external quantum efficiency (EQE)] of up to 44%.3 Shortly thereafter, the dye-sensitized solar cell (DSC) was invented based on mesoporous TiO2 and an organic electrolyte system containing the I3 -/I- redox couple.4,5 Since these pioneering days, DSC development has attracted much interest from academia and industry due to prospects of its greater performance, particularly under nonideal and diffuse light conditions, paired with relatively low cost and low energy manufacturing processes, and the options of light weight, flexibility, and partial transparency.6 While DSCs fabricated with traditional solvents and redox couples have reached efficiencies of 12.3%7 under Air Mass (AM) 1.5-type illumination, more recently, titania-based solid-state devices have been reported with laboratory efficiencies of 15%.8,9 This article describes the core materials required for successful assembly of DSC devices and their interaction in a photovoltaic device.
Main Components of Dye-sensitized Solar Cells
In general, the main components of DSCs are TiO2, ruthenium-based dyes, liquid electrolytes, platinum electrocatalysts, and silver inks. Each component material shows many interrelationships between different materials. If one DSC component is changed, e.g., the dye, other components such as TiO2 particle size, film thickness, or the electrolyte composition may need adjustment to ensure optimum system performance. Figure 1 shows the key components of a DSC, arranged from top to bottom, in the direction of incident light:
- Transparent substrate
- Layer of Transparent Conductive Oxide (TCO)
- Layer of mesoporous TiO2 having its surface covered by a monomolecular layer of dye
- Electrolyte solution filling the pores of the dyed TiO2 film and a thin layer between TiO2 and counter electrode
- Very thin coating of electrocatalyst, such as Pt
- Counter electrode substrate coated with a thin layer of TCO
- Perimeter seal and possibly fill hole seal if long-term stability is required
- Two current collector bus bars to maximize current collection efficiency (optional)
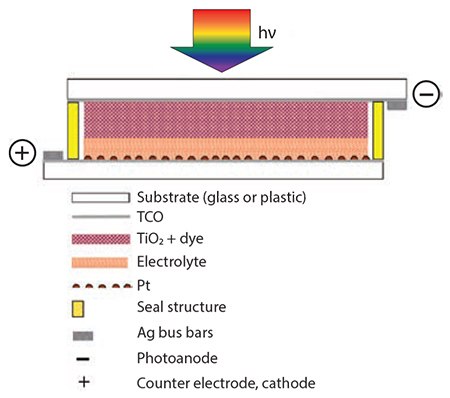
Figure 1.Schematic of the architecture and main components of a dye-sensitized solar cell.
TiO2 Pastes and Films
Titania is a widely available, nontoxic material used in everyday products such as toothpaste, sunscreens and paints. DSC technology requires the most active titania (anatase polymorph), in contrast to the paint industry where TiO2 has to be photochemically desensitized to minimize resin decomposition, yellowing, blistering, etc.10 Anatase TiO2 (Product No. 637254) is an n-type semiconductor and has a band gap of 3.2 eV, which corresponds to an optical onset of around 390 nm for light absorption. In traditional photovoltaic panels, solar-grade silicon requires 99.9999% purity (i.e., less than 1 ppm total impurities).11 In comparison, requirements for anatase TiO2 in DSC are less stringent but nevertheless important, and high-quality titania feedstock such as those used for the preparation of 18NR pastes (Product Nos. 791547 and 791555) have a phase purity of at least 99%. Other popular titania products for DSC research show a much lower phase purity, with considerable amounts of rutile as well as amorphous TiO2,12 thereby limiting the performance of the system.
Performance in a DSC not only depends on TiO2 chemical and phase purity, but on additional factors such as particle size and distribution, shape, surface hydroxylation, application methods, and sintering. Paste formulation, printing, drying and sintering parameters need to be carefully controlled to achieve the best optimized results. These parameters influence film morphology, overall porosity, and pore size distribution. 18NR-T (Transparent, Product No. 791547) and AO (Active Opaque, Product No. 791555) titania pastes have been used for record-breaking DSC work.8 Under recommended screen printing and firing conditions,18NR-T paste results in optically transparent 6–7 μM thick films and a film porosity of ca. 65%. Depending on the dye employed, 1 to 3 layers may be required. Alternatively, screens of larger or smaller mesh size can be employed to finely adjust overall film thickness.
Figure 2 shows an SEM image of a sintered 18NR-T film. The average particle size is ca. 20 nm. The nanoparticulate and mesoporous nature (i.e., pores with diameters between 2 and 50 nm) of titania electrodes in DSCs is important from at least five aspects:
- Optically to provide transparent films due to the particle size being significantly smaller than the wavelength of visible light.
- Electronically to avoid the formation of interfacial space charge layers, which would reduce device voltage. Since the particles are much smaller than the width of the space charge layer in a corresponding semiconductor (TiO2) based on much larger particles, no such layer is formed at the solid/electrolyte interface. Instead, electrons diffuse freely in the TiO2 conduction band. Their charge is effectively screened by positive ions in the electrolyte, which facilitates their transport through the TiO2 particle network.
- Mechanically and electronically to facilitate particle-to-particle connections at relatively low temperatures of ca. 500 °C. Larger grain titania particles can only be fused together at much higher temperatures, around 800 °C and above.
- High surface area for dye adsorption is required to ensure effective harvesting of the maximum possible incident light.
- Open porous structure, necessary for penetration of both dye molecules to anchor to titania and electrolyte ions to regenerate the dye once oxidized during device operation.

Figure 2.SEM of 18NR-T paste-based TiO2 film after sintering.
While transparent films offer the potential of semi-transparency of the final products for windows or skylight applications, they often do not allow for sufficient absorption of longer wavelength light. Therefore, some light scattering and internal reflection are desirable. This can be achieved in two ways, either by adding larger light scattering TiO2 particles to the titania paste or by adding a scattering layer on top of a transparent layer.
The former has advantages that the overall titania film thickness is not increased significantly; the added larger particles (up to 450 nm diameter in the case of 18NR-AO) not only contribute to performance through their light scattering effect, but actively through adsorption of dye and, hence, additionally enhance light conversion. Processing a single paste is also more convenient than a bi-layer structure. Addition of relatively large scattering particles leads to an increase of haze, which is defined according to Equation 1:
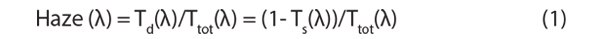
where Td(λ) is the diffuse (or scattered) transmittance at wavelength λ, Ts the specular transmittance, and Ttot the total transmittance. Haze of a 15 μM film based on 18NR-AO paste (Product No. 791555) is significantly above 99% over the entire visible spectrum. Device performance increases with haze at longer wavelengths (e.g., at 800 nm).13 18NR-AO paste is particularly suitable whenever highest conversion efficiency is required, especially when higher viscosity electrolytes are used. It facilitates the use of thinner titania films, which is advantageous in reducing tortuosity and improving electrolyte mobility.14 Employing 18NR-AO paste under the recommended screen printing and firing conditions results in white, opaque 7–8 μM thick films and a film porosity of ca. 55–60%. Similar to 18NR-T, 1 to 3 layers may be required depending on the nature of sensitizing dye, and screens of larger or smaller mesh size can be used to adjust overall film thickness.
When maximum output is sought, a scattering layer based on WER2-O reflector titania paste (Product No. 791539) can significantly enhance IPCE, particularly at longer wavelengths where dye absorption becomes weaker. WER2-O contains well-dispersed, largely DSC-inactive scattering particles of 150–250 nm diameter. A 3 μM thick layer leads to a completely opaque white film on top of a transparent active film.
Dyes
The dye can be considered the heart of DSCs. Its absorption spectrum and electronic coupling to n-type electron conductor determine absorption (ηabs) and electron injection efficiency (ηinj), which are two of the three key contributors to IPCE:

Considerable internal area is available for dye adsorption in titania films. The amount of dye adsorbed can be determined using chemical dye desorption and spectrophotometry methods.15 Typically for N719 (Product No. 703214), the amount of adsorbed dye is 1.1×107 mol per cm2 of projected area. Based on a film thickness (dTiO2) of 14 μM, this corresponds to a dye concentration within the film, ![]() = 7.8×10-5 mol per cm3. For a transparent film, the amount of absorbed light, i.e., the light not transmitted (=1-T), can be estimated from Equation 3:
= 7.8×10-5 mol per cm3. For a transparent film, the amount of absorbed light, i.e., the light not transmitted (=1-T), can be estimated from Equation 3:

If the extinction coefficient ε is, through adsorption on TiO2, not altered significantly from its εmax of value in solution (14,700 M-1cm-1 for N719), such an amount of dye can absorb 97.5% of the light at the absorption maximum. Note, however, that the absorption spectrum is red-shifted upon adsorption on TiO2. Assuming a surface roughness factor of ca. 1,300, each N719 molecule occupies an area of ca. 2 nm2. This figure is higher by 30–70% compared to the theoretically estimated ‘foot print’ of N316 (Product No. 703206), indicating that not all TiO2 sites are occupied or accessible.
The dyes N3, N719, and N749 display hydrophilic behaviors due to the presence of protonated and deprotonated carboxylic acid functionalities of all pyridine-based ligands. The other dyes display hydrophobic behavior, once anchored on TiO2, due to the presence of apolar chains on the non-anchoring bipyridine. All bipyridine dyes are charged balanced with respect to the ruthenium ion; the terpyridine dye is, however, anionic due to the presence of three isothiocyanate groups binding the ruthenium metallic center. The bipyridine-based dyes result in shades of red to maroon coloration; the terpyridine dye results in a dark green coloration which can be virtually black for non-transparent devices.
Aliphatic and other apolar side chains containing aromatic, electron rich, and donating moieties result in the following trends:
- Higher extinction coefficients due to the increased size of π-system so that generally lower quantities of dye (and ruthenium) are required per unit area
- Lower recombination, since I3 - access to the TiO2 surface is hindered by size and the dynamic motion of the side groups
- Increased electrostatic binding to the TiO2 surface due to higher ground state pKa
- Decreased charge on the dye attenuating the electrostatic repulsion between adsorbed dye units; thereby increasing the dye loading
- Increased stability of solar cells toward water-induced dye desorption
- Cathodic shift of the oxidation potential of these complexes, compared to that of the N3 sensitizer, which increases the reversibility of the ruthenium III/II couple, leading to enhanced stability17
In addition to the above mentioned advantages of extended aliphatic and conjugated side chains, the corresponding metal to ligand charge transfer (MLCT) is also expected to shift to a higher wavelength.18 The bathochromic effect is mainly controlled by the increase in conjugation between pyridines rather than by the lipophilic side chains. Also, modifying the number of conjugated electrons affects the molar absorptivity, which can be observed when comparing the bipyridinebased structures to the terpyridine one in Figure 4; the red shifts are relatively modest when going from N3 to C106 or even K19, as opposed to N3 versus N749.
/structures-of-bi--and-terpyridine-based (1).jpg)
Figure 3.Structures of bi- and terpyridine-based ruthenium dyes.

Figure 4. UV-VIS spectra for bi- and terpyridine-based ruthenium dyes.
Electrolytes
The electrolyte in DSC devices has several important functions:
- Internal charge transport to allow for current flow in an external circuit
- Dye regeneration following dye excitation and electron injection into TiO2 according to Equation 4:

- Diffusional equilibration of I3 -/I- concentration gradients created due to I3 - generation at the photoanode and partial I3 - depletion at the counter electrode.
Since DSC devices are electrochemical high-power devices with current densities of 20 mA/cm2 and beyond, adequate electrolyte conductivity and relatively fast diffusion are important for high device efficiency.
Table 2 compares electrolyte conductivity for EL-HPE (Product No. 791482), EL-HSE (Product No. 791466), and EL-HTE (Product No. 791458). Electrolyte conductivity generally decreases with increasing solvent viscosity and boiling point. It is important to note that direct resistive loss in DSC electrolytes are relatively minor compared to Ohmic loss through the TCO. An electrolyte conductivity of 10 mS/cm in a 1×1 cm cell with an internal gap of 40 μM results in a resistance of only 0.4 Ohm, while TCO resistance would typically be on the order of 10–18 Ohm for the same cell. On the other hand, electrolyte viscosity has a much more serious impact on losses due to concentration polarization, i.e., the establishment of a concentration gradient between anode and cathode.
Additionally, the electrolyte system has to be chemically and photochemically stable up to temperatures of 85 °C and even beyond in very hot climates. Unfortunately, the requirements of performance and stability are often diametrically opposed. High electrolyte conductivity and fast I3 - diffusion are generally achieved from solvents with a low viscosity and boiling point, such as acetonitrile. Such electrolyte systems, e.g., EL-HPE, are suitable to demonstrate high cell performance. Acetonitrile-based devices do not, however, offer prolonged stability of device output. For improved long-term stability, solvents with a higher boiling point and viscosity19 or even ionic liquid-based systems18,20 are required. Excellent long-term stability has been demonstrated for 100×100 to 150×150 mm DSC devices based on EL-HSE17 at 85 °C in the dark by the Fraunhofer Institute for Solar Energy System21 and under light and 85 °C by 3G Solar,22 respectively.
Platinum Paste
DSC devices based on the I3 -/I- redox shuttle require efficient electrochemical regeneration of photochemically produced I3 -. Reduction of I3 - to 3I- is a two-electron transfer process, a rather complex and relatively slow reaction that may involve a number of intermediates.23 Platinum (Pt) is the most efficient electrocatalyst so far, where quantities of 2–4 μg/cm2 correspond to an average thickness of 1–2 nm which is sufficient to provide an efficient device and transparency. It has been shown, however, that the most active Pt electrodes for electrochemical I3 - reduction are not flat and smooth, but rather based on Pt nanoclusters of ca. 5 nm average size distributed over the TCO surface.24 Such nanostructured layers can be prepared through thermal decomposition of H2PtCl6 at 380–420 °C.
PT-1 paste (Product No. 791512) has been formulated for easy and reproducible Pt deposition, primarily on TEC substrates, using high throughput screen printing to provide patterned deposition capabilities. This produces efficient counter electrodes for semitransparent as well as opaque DSC devices.
Silver Inks
High quality contacts and bus bars are important for minimizing resistive losses from current collection and from contact resistance to the conductive substrate. They are particularly important at higher light levels and for devices with an active area of greater than 1 cm2. Another requirement for electrical contacts is solderability for cabling purposes. Silver (due to its very high electrical conductivity, good resistance toward atmospheric corrosion, and favorable solderability) is the material of choice. Because of its high cost, it is important to achieve the specified conductivity at the lowest silver loading. For high-temperature compatible materials, such as for standard photovoltaic silicon wafers, glass frit-based silver pastes are employed, which are processed at temperatures above 500 °C. While such pastes can be used for glassbased DSC, they are not suitable in conjunction with polymer substrates for flexible devices, where polymer-based formulations are required.
The vast majority of polymer thick film (PTF) conductive silver inks consist of silver particles held together by an insulating organic resin or binder mixed with a solvent carrier. The inks can be printed onto a range of substrates using methods such as screen printing, flexographic printing, inkjet, etc., followed by drying in ovens. The drying process essentially removes the carrier solvent and conductivity is achieved as a result of point-to-point contact between the silver particles. However, at lower annealing temperatures (<200 °C), the residual nonconductive binders limit the achievable conductivity. Higher firing temperatures can help burn off residual binders and increase conductivity; however, it can have a detrimental effect on the substrate material and/or to adhesion between substrate and conductive film.
Unlike other conventional inks, DYAG silver inks (Product Nos. 791873, 791881, and 791903) are formulated to generate nanoscale silver particles as a result of a thermally induced reaction. “Chemically welded” bridges form between silver particles during the thermal process, resulting in a continuous metal track (Figure 5) which provides far superior particle-to-particle contacts compared to conventional inks. This enables DYAG silver inks to achieve higher conductivities at low curing temperatures and low silver content.
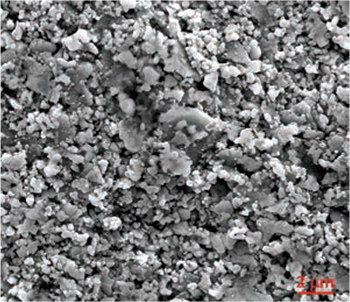
Figure 5.SEM of DYAG100 ink after drying and curing.
The DYAG series of inks has been designed to enable outstanding printability and excellent adhesion when applied on a variety of substrates, including polyesters and TCO-coated substrates, making them ideal for both flexible and rigid substrates. Table 3 shows a range of resistivity and application requirements of DYAG silver inks. These inks are not only suitable for DSC but also for organic PV (OPV) and, generally, for emerging printed electronics applications such as radio frequency identification tags, smart cards, OLEDS, electronic paper, etc., where relatively low temperature curing, combined with high conductivity, flexibility, and reliability are important.
Conclusions
While individual material optimization is key to achieve high efficiency, for instance maximizing light capture by controlling titania haze and dye extinction coefficient, the interaction of components, particularly the electrolyte, determine system stability. Not only is it important to have a clear understanding of the individual materials and their characteristics, but also being aware of the interactions within the combinations operating in a full DSC system is necessary for delivering the required array of final device properties. The widespread availability of such well-characterized and dependable products will facilitate consistent DSC research and enhance reproducibility and reliability of results. Improved research outputs will contribute to more rapid advances in DSC technology and assist in delivering tangible approaches to tackle future global energy requirements.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?