1.10317
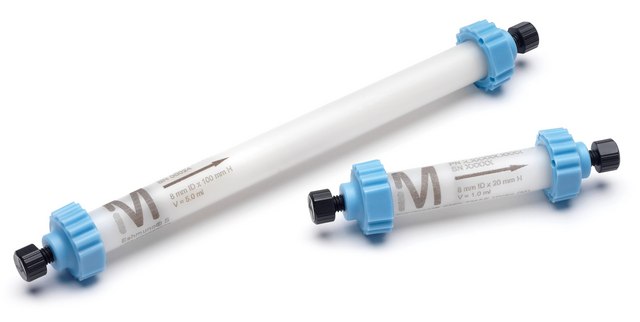
Fractogel® EMD BioSEC (S)
Synonym(s):
Fractogel® EMD BioSEC (S)
Select a Size
Select a Size
About This Item
Recommended Products
ligand
(Modified pore structure)
Quality Level
description
for size exclusion chromatography (SEC), suspension in 20% ethanol and 150 mM NaCl (20-40 µm)
product line
Fractogel®
parameter
100 cm/hr max. flow rate
8 bar pressure
matrix active group
Methacrylate
mean particle size
20-40 μm
transition temp
flash point 35 °C (calculated)
density
1.43 g/cm3 at 20 °C
application(s)
viral therapy
Related Categories
1 of 4
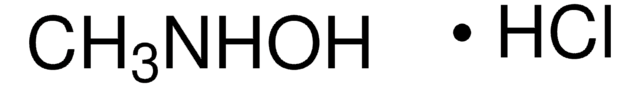
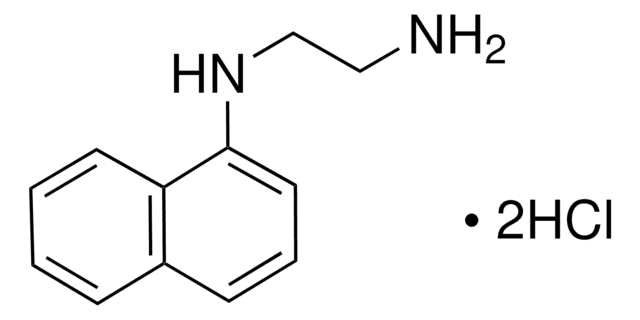
This Item | D163708 | 274992 | N9125 |
|---|---|---|---|
| Quality Level 200 | Quality Level 200 | Quality Level 200 | Quality Level 200 |
| mp 86-88 °C (lit.) | mp 112-115 °C | mp 130-133 °C (lit.) | mp 194-198 °C (dec.) (lit.) |
General description
Features and Benefits
- Effectively remove dimer and high molecular weight aggregates and complete an efficient polishing of blood plasma factors
- Determine apparent molecular weights of proteins
- Move predictably and efficiently from bench scale to manufacturing scale processing
- Create a robust, consistent, and transferable process
Packaging
Analysis Note
Microscopic evaluation: Uniform sphericalparticles, noagglomerates, nofines
Extractable matter (water): ≤ 0.03 %
Cerium: ≤ 1 µg/g
Pressure drop(column: ID=1.6 cm, L=10 cm at 5 ml/min): ≤ 5.0 bar
Particle size (d10): 20 - 28 µm
Particle size (d50): 24 - 34 µm
Particle size (d90): 28 - 38 µm
Colony forming units (TAMC + TYMC): ≤ 100 CFU/ml
Endotoxins: ≤ 1.00 EU/ml
Function test (b:a): ≤ 0.7
function test (c:d): ≤ 0.05
Functional Test: Separation of bovine serum albumin, ovalbumin and cytochrom C
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
95.0 °F
Flash Point(C)
35 °C
What is the Emprove® Program?
The Emprove® Program is a system providing comprehensive and thorough documentation of our filters and single-use components, pharma raw materials, and starting materials. Four document types are included with an Emprove® Program subscription:
Free of charge - Sign in to download
Free of charge - Sign in to download
Available by subscription or for a fee
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Influenza vaccines are commonly made using egg-based and cell-based manufacturing strategies. Find step-by-step information on the manufacturing process for each method.
A custom-designed cost model is used to explore the economics of vaccine manufacturing across several different modalities including mRNA. The model enables greater process understanding, simulates bottlenecks, and helps to optimize production efficiency.
Learn more one the attenuated viral vaccines manufacturing process: cell culture, clarification, nuclease treatment, chromatography, and sterile filtration.
This technical article breaks down the steps of upstream and downstream bioprocessing and formulation of virus-like particle vaccines.
Related Content
This technical article breaks down the adenovirus vaccine manufacturing process and provides a case study on developing an accelerated and cost-effective single-use adenoviral vector vaccine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service