GenElute™ Bacterial Genomic DNA Kit Protocol
(NA2100, NA2110, NA2120)
GenElute™ Bacterial Genomic DNA Kit Description
Our GenElute™ Bacterial Genomic DNA Kit provides a simple and convenient way to isolate pure DNA from a variety of cultured bacteria. The kit contains all of the reagents needed to isolate and purify genomic DNA from gram-negative bacteria. For most gram-positive bacteria, the kit must be used in conjunction with the optional lysozyme (L4919), to effectively lyse the thick peptidoglycan cell walls. A Gram-Positive Lysis Solution is provided as a diluent for preparing the lysozyme stock solutions.
The GenElute™ kit combines the advantages of a silica-based system with a microspin format and eliminates the need for expensive resins, alcohol precipitation, and hazardous organic compounds such as phenol and chloroform. The bacteria are lysed in a chaotropic salt-containing solution to ensure the thorough denaturation of macromolecules. The addition of ethanol causes the DNA to bind when the lysate is spun through a silica membrane into a microcentrifuge tube. After washing to remove the contaminants, the DNA is eluted in 200 μL of a Tris-EDTA solution.
The expected yield of genomic DNA will vary depending on the cell density of the bacterial culture and the bacterial species and strain used. Appendix 2 lists the typical yield of genomic DNA purified from some gram-negative and gram-positive bacteria. DNA purified with the GenElute™ kit has an A260/ A280 ratio between 1.6 and 1.9 and can be up to 50 kb in length. This DNA is ready for downstream applications such as restriction endonuclease digestions, PCR, and Southern blots.
Equipment and Reagents Required But Not Provided
- 37 °C water bath or heating block
- 55 °C water bath or heating block
- Pipette tips (aerosol barrier recommended)
- 1.5 mL microcentrifuge tube for lysis
- Microcentrifuge (2 mL tube, rotor equipped)*
- Ethanol (95%–100%), Catalog Nos. E7023, E7148, or 459836
- Molecular Biology Reagent Water, Catalog No. W4502
- Lysozyme, Catalog No. L4919 (for gram-positives only)
- Mutanolysin, Catalog No. M9901 (for Streptococcus species only)
- Lysostaphin, Catalog No. L7386 (for Staphylococcus only)
Note: To ensure proper fit of all tubes, a 24-place rotor is recommended. If you are using a 36-place rotor, we recommend using every other place for proper tube fit.
Storage and Stability
Store the kit at room temperature. If any kit reagent forms a precipitate, warm at 55–65 °C until the precipitate dissolves and allow to cool to room temperature before use.
Preparation Instructions
- Preheat a water bath or heating block to 55 °C
For use with both gram-positive and gram-negative bacteria.
- Preheat a water bath or heating block to 37 °C
For use with gram-positive bacteria only.
- Thoroughly Mix Reagents
Examine reagents for precipitation. If any reagent forms a precipitate, warm at 55–65 °C until the precipitate dissolves and cool to room temperature before use.
- Dilute Wash Solution Concentrate
Dilute the concentrate with 10 mL (10 prep package), 80 mL (70 prep package), or 360 mL (350 prep package) of 95–100% ethanol. After each use, tightly cap the diluted Wash Solution to prevent the evaporation of the ethanol.
- Reconstitute Proteinase K
Dissolve the powder in one bottle of Proteinase K in water to obtain a 20 mg/mL stock solution, according to Table 1. The Proteinase K solution can be stored for several days at 2–8 °C. For longer-term storage, the unused portion of the
solution may be stored in aliquots at –20 °C until needed. This product as supplied is stable at room temperature.
Note: The Proteinase K solution must be added directly to each sample every time. Do not combine the Proteinase K Solution and Lysis Solution for storage.
- Prepare Lysozyme Solution (for gram-positive bacteria only)
Prepare a 2.115 × 106 unit/mL stock solution of lysozyme (L4919) (approximately 45 mg/mL) using the included Gram-Positive Lysis Solution (L7539) as the diluent. For example, to make 1 mL of Lysozyme Solution, dissolve 2.115 × 106 units of lysozyme in 1 mL of Gram-Positive Lysis Solution.
Pipette the mixture up and down or vortex to dissolve the lysozyme (see note below). For each DNA preparation to be performed, 200 μL of Lysozyme Solution is required. Make extra solution to account for pipetting error. The Lysozyme Solution should be used on the day of preparation.
Note: Lysozyme may dissolve more readily by pipetting the mixture up and down as opposed to vortexing. Excessive vortexing may cause foaming. The lysozyme may not dissolve readily, in which case it does not need to be completely dissolved prior to use. Genomic DNA yields will not be affected as the lysozyme will dissolve during the 37 °C incubation.
Procedure
If minimally sheared genomic DNA is desired in downstream applications, e.g., if using the end product for long amplification PCR, mix with gentle pipetting or inversion until homogeneous instead of vortexing in the procedure that follows. See Appendix 1 to convert g-force to RPM.
A. Gram-Negative Bacterial Preparation
1a. Harvest Cells
Pellet 1.5 mL of an overnight bacterial broth culture by centrifuging for 2 minutes at 12,000–16,000 × g. Remove the culture medium completely and discard.
Note: If bacteria are propagated in rich media such as Terrific broth (T9179) it will be necessary to reduce the volume of starting material to 0.5 mL of an overnight bacterial broth culture to avoid overloading the GenElute™ columns. See Appendix 2 for more information.
2a. Resuspend Cells
Resuspend the pellet thoroughly in 180 μL of Lysis Solution T/Buffer STL for GenElute™ Mammalian Genomic DNA Kit (B6678). If residual RNA is not a concern, continue with step 3a.
Optional RNase A treatment: If RNA-free genomic DNA is required, add 20 μL of RNase A Solution (R6148), mix, and incubate for 2 minutes at room temperature, then continue with step 3a.
3a. Prepare for Cell Lysis
Add 20 μL of the Proteinase K solution to the sample. Mix and incubate for 30 minutes at 55 °C.
4a. Lyse Cells
Add 200 μL of Lysis Solution C (B8803), vortex thoroughly (about 15 seconds), and incubate at 55 °C for 10 minutes.
A homogeneous mixture is essential for efficient lysis.
Continue with step 5.
B. Gram-Positive Bacterial Preparation
1b. Prepare Lysozyme Solution Using Lysozyme from Chicken Egg White (L4919)
Prepare a 2.115 × 106 unit/mL stock solution of lysozyme as described under Preparation Instructions. For each DNA preparation to be performed, 200 μL of Lysozyme Solution is required. Prepare extra solution to account for pipetting error.
Note: If working with Staphylococcus species, supplement the Lysozyme Solution with 200 units/mL of lysostaphin (L7386). For Streptococcus species, supplement the Lysozyme Solution with 250 units/mL of mutanolysin (M9901).
2b. Harvest Cells
Pellet 1.5 mL of an overnight bacterial broth culture by centrifuging for 2 minutes at 12,000–16,000 × g. Remove the culture medium completely and discard.
Note: If bacteria are propagated in rich media such as Terrific broth (T9179), it will be necessary to reduce the volume of starting material to 0.5 mL of overnight bacterial broth culture to avoid overloading the GenElute™ columns. See Appendix 2 for more information.
3b. Resuspend Cells
Resuspend the pellet thoroughly in 200 μL of Lysozyme Solution (prepared in step 1b) and incubate for 30 minutes at 37 °C.
Optional RNase A treatment: If residual RNA is not a concern, continue with step 4b. If RNA-free genomic DNA is required, add 20 μL of RNase A Solution and incubate for 2 minutes at room temperature, then continue with step 4b.
4b. Lyse Cells
Add 20 μL of the Proteinase K solution to the sample, followed by 200 μL of Lysis Solution C (B8803). Vortex thoroughly (about 15 seconds) and incubate at 55 °C for 10 minutes. A homogeneous mixture is essential for efficient lysis. Continue with step 5.
DNA Isolation from Gram-Positive and Gram-Negative Bacteria
This is a continuation of the procedure from the lysates prepared in steps 1–4a and/or steps 1–4b.
5. Column Preparation
Add 500 μL of the Column Preparation Solution to each pre-assembled GenElute™ Miniprep Binding Column (with a red o-ring, not to be confused with other GenElute™ kits) seated in a 2 mL collection tube. Centrifuge at 12,000 × g for 1 minute. Discard the eluate.
Note: The Column Preparation Solution maximizes binding of DNA to the membrane resulting in more consistent yields.
6. Prepare for Binding
Add 200 μL of ethanol (95–100%) to the lysate and mix thoroughly by vortexing for 5–10 seconds. A homogeneous mixture is essential.
7. Load Lysate
Transfer the entire contents of the tube into the binding column. Use a wide bore pipette tip to reduce shearing the DNA when transferring the contents into the column. Centrifuge at ≥ 6500 × g for 1 minute. Discard the collection tube containing the eluate and place the column in a new 2 mL collection tube.
8. First Wash
Add 500 μL of Wash Solution 1 (W0263) to the column and centrifuge for 1 minute at ≥ 6500 × g. Discard the collection tube containing the eluate and place the column in a new 2 mL collection tube.
9. Second Wash (Important Reminder: Verify that ethanol has been added to the bottle of Wash Solution Concentrate.)
Add 500 μL of Wash Solution to the column and centrifuge for 3 minutes at maximum speed (12,000–16,000 × g) to dry the column. The column must be free of ethanol before eluting the DNA.
Centrifuge the column for an additional 1 minute at maximum speed if residual ethanol is seen. You may empty and re-use the collection tube if you need this additional centrifugation step. Finally, discard the collection tube containing the eluate and place the column in a new 2 mL collection tube.
10. Elute DNA
Pipette 200 μL of the Elution Solution (B6803) directly onto the center of the column; centrifuge for 1 minute at ≥ 6500 × g to elute the DNA. To increase the elution efficiency, incubate for 5 minutes at room temperature after adding the Elution Solution, then centrifuge.
Optional: A second elution can be collected by repeating step 10 with an additional 200 μL of Elution Solution and eluting into a new 2 mL collection tube or into the same 2 mL collection tube as used for the first eluate. The yield can be improved by 20–50% when performing a second elution.
The eluate contains pure genomic DNA. For short-term storage of the DNA, 2–8 °C is recommended.
For longer-term storage, –20 °C is recommended. Avoid freezing and thawing, which causes breaks in the DNA strand. The Elution Solution will help stabilize the DNA at these temperatures.
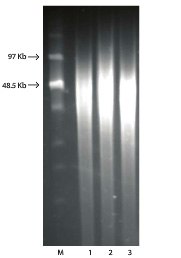
Figure 1. PFGE of Bacterial gDNA isolated with GenElute™ Bacterial gDNA Kit.
Purified genomic DNA was isolated from various bacterial species using the GenElute™ Bacterial Genomic DNA kit. A 1 μg aliquot of DNA from each respective bacterial sample was resolved on a 1% agarose gel in 0.5X TBE at 150 volts for 16 hours using a BioRad CHEF DRII system. The initial pulse time was 2 seconds, the final pulse time was 13 seconds, the start ratio was 1.0, pump speed was set at 70, and PFGE was carried out at 4 °C. M represents the 0.1–200 kb Pulse marker (Cat. No. D2291).
Lanes
- E. coli
- P. fluorescens
- B. subtilis
Results
The concentration and quality of the genomic DNA can be determined by spectrophotometric analysis and agarose gel electrophoresis. Dilute the DNA in TE Buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0–8.5) and measure the absorbance at 260 nm, 280 nm, and 320 nm using a quartz microcuvette. The absorbance at 260 nm and 280 nm should be between 0.1 and 1.0 (or within the linear range of your spectrophotometer). The 320 nm absorbance is used to correct for background absorbance. An absorbance of 1.0 at 260 nm corresponds to approximately 50 μg/mL of double-stranded DNA. The A260–A320/A280–A320 ratio should be 1.6–1.9.
The size and quality of the DNA can be determined by agarose gel electrophoresis.1 A gel containing 0.8% agarose (A9539) in 0.5X TBE Buffer (T6400) works well for the resolution of genomic DNA. The DNA can be visualized by staining with an intercalating dye such as ethidium bromide (E1510) and measured against a known DNA marker such as Lambda DNA Hind III digest (D9780). The genomic DNA should migrate as a single, high molecular weight band with very little evidence of shearing. A
more precise determination of the size of the DNA can be made by pulsed-field gel electrophoresis.2
Troubleshooting
Appendix 1
Note: All centrifugation speeds are given in units of g. Please refer to Table 2 for information on converting g-force to RPM. If centrifuges/rotors for the required g-forces are not available, use the maximum g-force possible and increase the spin time proportionally. Spin until all liquid passes through the column.
See table above for spin speeds in RPM for selected common centrifuges and rotors. The correct RPM for unlisted rotorscan be calculated using the formula:

Precautions and Disclaimer
The GenElute™ Bacterial Genomic DNA Kit is for R&D use only, not for drug, household, or other uses. Please consult the Safety Data Sheet (SDS) for information regarding hazards and safe handling practices.
GenElute is a trademark of Sigma-Aldrich Co. LLC.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?