G005818
LC/MS (TOF) Analysis of Antiarrhythmic Drugs and Metabolites in Plasma on Ascentis® Express HILIC after Sample Prep using HybridSPE®-Phospholipid
application for HPLC
Select a Size
¥2,486.94
Available to ship on2025年4月23日Details
Select a Size
About This Item
¥2,486.94
Available to ship on2025年4月23日Details
technique(s)
HPLC: suitable
test parameters
sample preparation: SPE (Solid Phase Extraction)
sample/matrix: rat plasma stabilized with K2EDTA was spiked directly from stock standard to a level of 400 ng/mL
SPE tube/cartridge: HybridSPE-Phospholipid 96-Well Plate, 50 mg/2 mL (575656-U)
sample addition: apply 100 μL of plasma to plate, followed by 300 μL of 1% formic acid acetonitrile. Agitate via vortex for 4 min
elution: place on vacuum manifold and apply 10” Hg vacuum for 4 minutes. Collect filtrate and analyze directly.
column: Ascentis Express HILIC, 10 cm x 2.1 mm I.D., 2.7 μm particles (53939-U)
mobile phase: [A] 5 mM ammonium formate; [B] 5 mM ammonium formate in acetonitrile; (5:95, A:B, pH 7.0 with formic acid)
flow rate: 0.4 mL/min
pressure: 1305 psi (90 bar)
column temp.: 35 °C
detector: ESI(+), full scan, m/z 100-1000
injection: 0.5 μL
sample: plasma extract, analyte concentration of final sample work up is equivalent to 100 ng/mL
suitability
application for HPLC
application(s)
clinical
1 of 4
This Item | 17936 | C0225000 | 17938 |
|---|---|---|---|
| assay ≥99% (HPLC) | assay ≥97.0% (HPLC) | assay - | assay ≥99.0% (HPLC) |
| form solid | form crystals | form solid | form powder or crystals |
| storage temp. −20°C | storage temp. −70°C | storage temp. −20°C | storage temp. −20°C |
| Quality Level 300 | Quality Level 200 | Quality Level - | Quality Level 200 |
| Gene Information human ... VDR(7421) | Gene Information human ... VDR(7421) | Gene Information human ... VDR(7421) | Gene Information human ... VDR(7421) |
Analysis Note
Legal Information
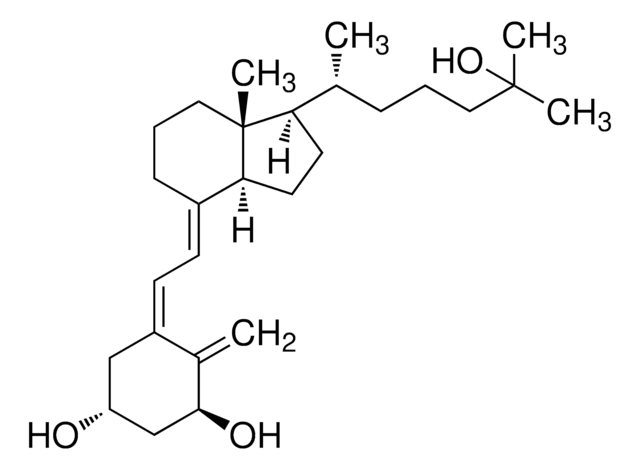
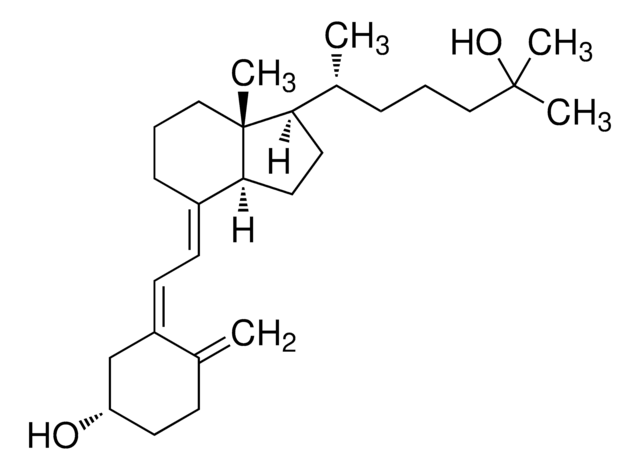
Analyte
SPE tube or plate
accessory
analytical column
standard
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service