Preservation of Moisture-Sensitive Chemical Reagents
Preservation of Moisture-Sensitive Reagents and Molarity Determinations

To help preserve reagent quality, many of our air- and moisture-sensitive reagents are packaged under nitrogen or argon in crown-cap bottles, with a 6 mm diameter hole in the crown-cap and a PTFE faced rubber liner under the crown-cap, for transfer of reagent using a syringe or cannula. When the syringe needle or cannula is withdrawn, the rubber liner reseals to prevent inflow of moist air, which would react with the reagent. Syringe techniques have the disadvantage that a partial vacuum is created in the bottle by removal of reagent, which is sufficient to pull (moist) air through the pierced crown-cap liner. To overcome this disadvantage, provide a blanket of dry nitrogen or inert gas over the crown-cap, so that it is dry nitrogen or inert gas that replaces the reagent in the bottle. The following products will enable you to provide an effective blanket of dry nitrogen or inert gas over our crown-cap bottles.
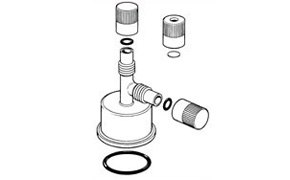
Sure/Seal™ Septum Inlet Transfer Adapter
This adapter screws over the crown cap, seating on a large o-ring that fits the bottle neck for an air-tight seal. Dry nitrogen or inert gas may be introduced by the side arm, then the cap on the vertical arm removed and replaced with either a fold-over rubber septum or the septum cap provided. Syringe techniques may be used to remove the desired amount of reagent, with the syringe needle passing vertically through the adapter and crown-cap. The reagent removed is replaced by dry nitrogen or inert gas from within the adapter, protecting the contents of the bottle from air or moisture.
Product No. Z407186
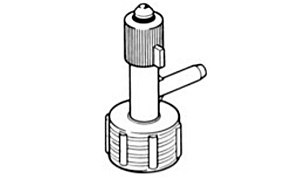
Oxford Sure/Seal™ Storage Valve Cap
This valve cap works on a similar principle to the transfer adapter described above, with the additional feature of a plunger cap that provides better protection for long term storage. This valve cap screws over the crown cap, onto the bottle neck for an air-tight seal. Dry nitrogen or inert gas may be introduced by the side arm and the plunger cap on the vertical arm removed and replaced with a fold-over rubber septum.
Product No. Z406260
Syringe techniques may be used to remove the desired amount of reagent, with the syringe needle passing vertically through the valve cap and crown-cap. The reagent removed is replaced by dry nitrogen or inert gas from within the valve cap, protecting the contents of the bottle from air or moisture. After removing bottle contents, the plunger cap is replaced, before the dry Nitrogen or inert gas supply is disconnected. The plunger part is long enough to pass by the side arm, effectively sealing the bottle with dry nitrogen or inert gas over the crown-cap, providing an effective positive seal for long term storage of the bottle.
Info on titration methods to determine the molarity:
As the concentrations of commercial solutions of e.g. n-BuLi or t-BuLi vary appreciably, and change during storage time, it is always recommended to determine the exact molarity of the solution before use.1 There can be found several methods for this in literature:
The classical Gilman double titration method is described in detail by Wakefield.2 A recommended method for routine analysis involves titration of the reagent with s-butyl alcohol using 1,10-phenanthroline or 2,2`-biquinoline (Product No. 35020) as indicator.3 A variety of other methods have been described as well.4
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?