BRAND® Insert 2in1 Supports the Cultivation of Reconstructed Human Epidermis (RhE) used for Skin Corrosion Tests (OECD TG 431)
Introduction
Reconstructed Human Epidermis (RhE) is used as an alternative in vitro test system partially able to replace tests on laboratory animals and provide data that may be more predictive for humans when compared to animal testing. For these reasons 3D tissue models become more and more attractive not only for research but also in the context of regulatory hazard identification of irritant and corrosive chemicals (OECD TG 431*). However, to be used for regulatory decision making, a validated RhE must meet certain quality criteria to reliably distinguish the different hazard potentials of chemicals. Here we show that human derived keratinocytes cultivated in the BRAND® Insert 2in1 differentiate into RhE models using the standard cultivation procedure including a submerged and air-liquid interphase condition. The RhE reproducibly determines the corrosive potential of the categorized chemicals.
*OECD Test Guideline for testing chemicals 431: In vitro skin corrosion: reconstructed human epidermis (RHE) test method; 2015
Methods
Cell culture
Reconstructed human Epidermis was generated using normal human keratinocytes seeded in BRAND® Insert 2in1 or cell culture inserts from another manufacturer in a density of 2*105 cells/cm² (125.000 cells in 200 µL per insert). For submerged and air-liquid interphase (ALI) cultures both insert types were placed standing on the bottom of culture plates. BRAND® Inserts featured a plasma-treated (cellGrade™ plus) polycarbonate membrane with a pore size of 0.4 µm and a culture area of approximately 0.6 cm². After submerged cultivation ALI-culture was initiated to induce keratinocyte differentiation into the multilayered epidermal model and finally exposed to chemicals. MTT assay and test substance application was performed according to the SOP for epiCS® In Vitro Skin Corrosion (CellSystems®).
Test substances
For each exposure time and chemical 3 RhE models were used for in vitro skin corrosion testing. The test chemicals applied were phosphate buffered saline (PBS) (negative control), 8N KOH (positive control), 4-(Methylthio)-benzaldehyde, lactic acid and formic acid. RhE mean viability was determined for each test chemical after 3 and 60 min of exposure and normalized to the mean viability of negative controls at the corresponding time point.
Results
Morphology
The RhE models were fixed with Bouin’s Solution and subsequently cryo-embedded. Following cross sections of the RhE samples were stained with hematoxylin and eosin and subjected to histological imaging. The RhE models show the typical layers of native skin with a multilayered corneal layer (stratum corneum).
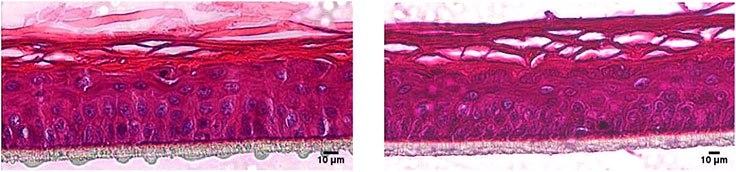
Figure 1.Hematoxylin/eosin staining of RhE models cultivated in the cell culture Insert 2in1 (right) and in an insert from another manufacturer (left). Human derived keratinocytes develop a stratified epidermis with a multilayered stratum corneum.
Barrier function test (EC50)
To determine whether the stratum corneum of RhE models cultured in different inserts developed a proper barrier function cultures were exposed to PBS and the benchmark chemical Triton X-100 for 60 min. After the exposure, RhE models were incubated in presence of MTT vital dye. Quantification of the metabolic activity was determined by measuring the optical density of the reduced MTT-dye at 570 nm wave length. Data indicates a distinct barrier function of the stratum corneum as the mean viability of the cultures was not reduced by more than 50 % at the given exposure time.
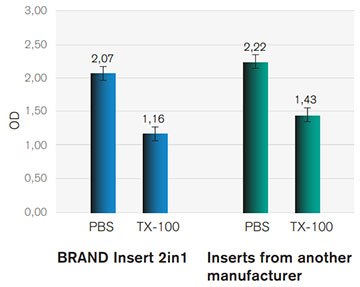
Figure 2.Viability of RhE exposed to PBS or Triton X-100 (TX-100). Data shows the optical density at 570 nm (OD) of isopropanol extracted formazan from tissue cultures.
First, MTT assay derived viability of RhE models was determined for 3 and 60 minutes using PBS. Measurements show that the viability of RhE models within the two insert types is comparable. However, tissue cultures from the BRAND insert 2in1 generated data with reduced standard deviations at 3 and 60 min of PBS exposure when compared to tissue culture grown in the competitor insert (Table 1).
To test whether the RhE models cultivated in BRAND® insert 2in1 can also be used to distinguish the corrosive potential of chemicals, RhE models were exposed to a set of classified substances. In parallel, the same chemicals were applied to RhE models cultivated in the insert from another manufacturer used in chemical hazard identification context before. The mean viability of treated RhE models was normalized to viability data of the negative control (NC).

Figure 3.Comparison of corrosive potential of different chemicals. NC, negative control; PC, positive control. Data show mean viability of 3 RhE per condition with standard deviation. Viability was determined by MTT assay. Optical density of isopropanol extracted formazan was measured in microplate spectrophotometer at 540 -570 nm.
The presented data show that the BRAND® Insert 2in1 with PC membrane and a pore size of 0.4 µm supports the differentation of normal human keratinocytes to a stratified epidermis model. Tissue models from the two inserts predicted 4-(Methylthio)- benzaldehyde as non corrosive chemical because viability is not reduced by 50 % after 3 min and 60 min of exposure when compared to NC. Formic acid is predicted to be corrosive because viability of tissue models from both inserts is reduced by more than 50% and more than 85% after exposure for 3 min and 60 min, respectively. Lactic acid is a corrosive substance of subcategory 1B/1C, which is shown by a viability higher than 50 % after 3 min and lower than 15 % after 60 min exposure, respectively. The not significant difference within the 3 min exposure with the BRAND® insert may be due to the small number of measurements.
Conclusion
BRAND® 2in1 Inserts are equally suitable to produce RhE as inserts from other manufacturers. This was shown by the comparison of H&E stained histological slides with the mulilayered stratified epidermis (Figure 1) and the integrative growth of the keratinocytes with a functional barrier function was demonstrated by EC50 data (Figure 2). Using proven chemicals for the OECD corrosion test with RhE, we could measure data comparable with inserts of another manufacturer. The BRAND® Insert 2in1 is a promising tool for use in corrosion tests and a step forward to avoid animal testing and gather data much more transferable to humans than animal testing ever will be.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?