MaxGel™ ECM Hydrogel: An In Vitro Human Cell Derived Basement Membrane Extract for 3D Cell Culture Applications
What is MaxGel™ ECM?
MaxGel™ ECM is an in vitro derived human basement membrane extracts (BME) that provides a rich three-dimensional environment to promote cellular proliferation for multiple cell types. MaxGel™ ECM contains extracellular matrix components including collagens, laminin, fibronectin, tenascin, elastin, a number of proteoglycans and glycosaminoglycans. MaxGel™ ECM is a human-based alternative to EHS murine sarcoma cell basement membrane extracts (BME) and is produced from the in vitro co-culture of human fibroblasts and human epithelial cells. In contrast to basement membrane extracts (BME) isolated from Engelbreth-Holm-Swarm (EHS) murine tumors that contains high concentrations of growth factors (especially TGF-β), MaxGel™ ECM contains levels of TGF-β that are very similar to the levels seen in vivo (<1 mg/mL). The cell-cultured derived ECM effectively reproduces the cooperative interaction of epithelia and mesenchyme during development and in organotypic cell culture of skin. Human MaxGel™ ECM promotes cell growth and migration and has been shown to support the proliferation of many cell types, including neural stem cells, neurons, glia, astrocytes, fibroblasts, hepatocytes and keratinocytes.
MaxGel™ ECM Publications
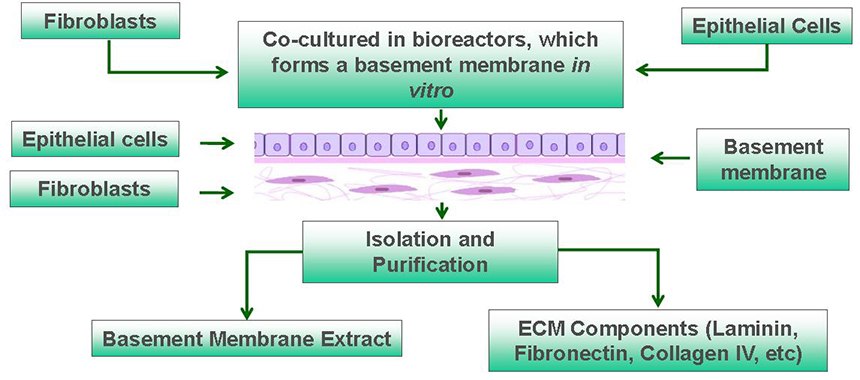
Figure 1. Overview of Human MaxGel™ ECM Hydrogels. Scalable isolation and purification of MaxGel™ ECM hydrogels from cocultures of human fibroblasts and epithelial cells in bioreactors.
How to use MaxGel™ ECM as a Coating Material?
This cell culture derived human BME is not denatured nor chloroform sterilized. It provides for a more tissue-like environment, due to the non-denatured acid-soluble collagens, which form fibrils through a self-assembly process at neutral pH. MaxGel™ ECM is supplied as a ~1 mg/mL solution in Dulbecco's Modified Eagle's Medium (DMEM) with penicillin-streptomycin added and is compatible with all culture media. Remove the product from the freezer, thaw at 2–8 °C or on ice just prior to use, and keep the product cold until coating.
Thin Coating Procedure
For monolayer culture or when a thin coating of BME is desired, dilutions of as high as 1:100 can be used. An empirically determined dilution should be performed depending on the particular application. For example, human embryonic stem cells and keratinocytes grow well when cultured on a 1:100 dilution of MaxGel ECM.
- Dilute the BME in cold medium.
- Plate the appropriate volume of the diluted BME solution in a tissue culture plate and allow it to incubate for 2–4 hours at 37 °C in a humidified 5% CO2 incubator.
- Carefully aspirate the remaining solution before plating cells.
- Air-dry for 30 minutes at room temperature.
- Plate cells as desired.
MaxGel™ ECM Cell Applications
Stem Cell Culture
Stem cell research has demonstrated the need for human ECM proteins in cell culture as 3D support matrices for stem cells. The proteins provide the cues necessary to expand stem cell in culture and to guide them through the process of differentiation into many cell types to treat different diseases, such as Pancreatic islets for diabetes and neuronal cells for repair of neurological disorders.
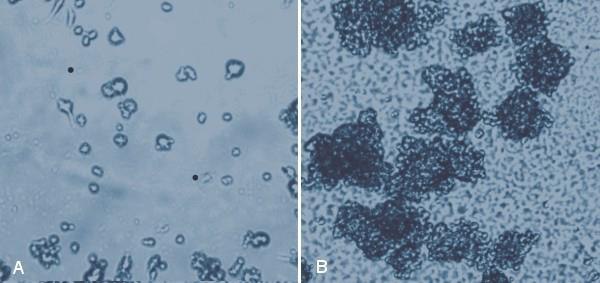
Figure 2. MaxGel™ ECM provides an excellent substrate for neural stem cells. Rat neural stem cells (SCR022) were cultured on tissue culture plastic (A) where they formed few neurospheres after 24 hours, but showed excellent neurosphere formation on 1% (w/v) MaxGel™ ECM after 24 hours (B).
Primary Cell Culture
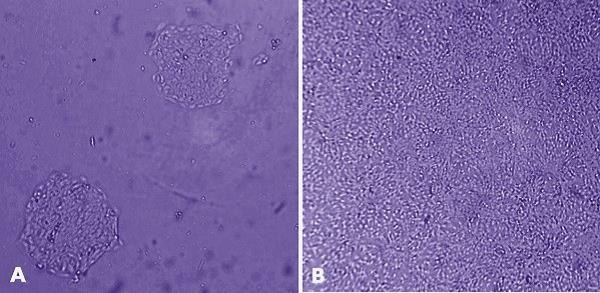
Figure 3. MaxGel™ ECM enables improved expansion of adult keratinocytes. HaCaT cells (derived from human adult skin keratinocytes) were grown for 24 hours after plating on tissue culture plastic (A) and on 1% (w/v) human MaxGel™ (B), which demonstrates that HaCaT cells proliferate better on MaxGel™.
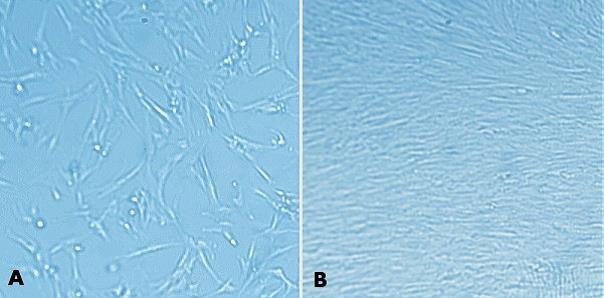
Figure 4. MaxGel™ ECM enables improved expansion of MRC-5 cells. MRC-5 human fetal lung fibroblasts (05072101) were grown for 24 hours after plating on tissue culture plastic (A) and on 1% (w/v) MaxGel™ ECM (B). As seen with other cells, MRC-5 cells propagate better on MaxGel™ ECM.
Xenograph Cancer Cell Models
The ability of MaxGel™ to increase cell proliferation and tumor formation and improve subsequent delivery of therapeutic stem cells for the treatment of prostate cancer was studied. In order to influence tumor formation, xenografts were induced in nude mice by subcutaneous inoculation of PC3 human prostate cancer cells (90112714) with and without MaxGel™.
Mesenchymal stem cells were engineered with the yeast cytosine deaminase gene that converts non-toxic 5-fluorocytosine into the highly toxic anti-tumor 5-fluorouracil, which selectively targets tumor cells. This engineered stem cell line (CD-MSC) was delivered into nude mice after tumor formation with and without MaxGel™ to determine the degree of tumor regression. MaxGel™ promoted significantly greater proliferation of tumor cells in a xenograft tumor model in nude mice. Even in the presence of this increased tumor mass, MaxGel™ provided a superior support matrix for therapeutic stem cell growth and differentiation, resulting in greater tumor necrosis than using therapeutic stem cells alone.

Figure 5. MaxGel™ ECM support in vivo xenograph cancer models. After 14 days, MaxGel™ ECM (1 mg/mL) caused significantly larger tumors in mice than did PC3 tumor cells alone. Following delivery of 2 x 10 CD-MSC delivered intravenously into nude mice (n=9 each group) either alone or in conjunction with MaxGel™ ECM, it was determined that MaxGel™ was able to cause a significant reduction of tumor size due to its ability to augment delivery of CD-MSC to the tumor site and facilitate tumor killing by the therapeutic stem cells.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?