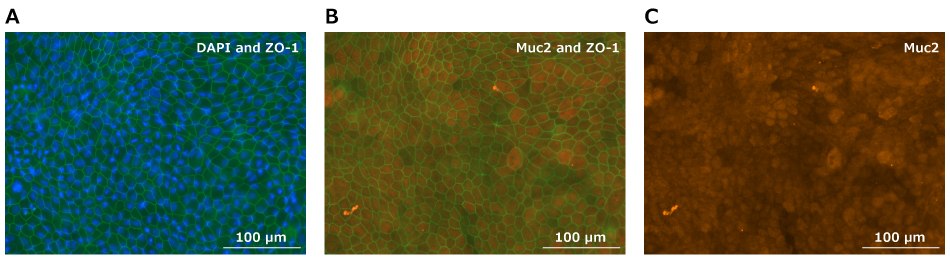
Growing Small Intestine Organoid-Derived Monolayers using Millicell® Inserts
What is 2.5D Cell Culture?
In 2.5D cell culture, researchers grow a 2D monolayer of cells on physical scaffolds, such as cell culture inserts, with a biological coating or synthetic polymer. This encourages the growth of differentiated structures that are most often associated with 3D cultures and enhances attachment and differentiation.
Although 3D organoid cultures offer several advantages over 2D cellular monolayer models, including better reflection of physiological features and biological complexity, 3D cultures are not amenable to applications such as trans-epithelial electrical resistance (TEER) to assess barrier integrity, drug influx/efflux, migration and invasion, and wound healing assays. To address these important questions, organoids are dissociated into single cells and cultured on membranes as monolayers in 2.5D cultures.
The small intestine is the site of drug absorption and is a highly relevant model for drug toxicity, permeability, and influx/efflux. In 3D in vitro culture, intestinal organoids form bubble-like structures with the inside (apical side) of the structure corresponding to the lumen (side of absorption) and the outside (basolateral side) corresponding to the blood vessel-facing area. This configuration makes any treatment with compounds that are absorbed on the apical side challenging. If the same organoid line is cultured on a transmembrane insert, however, a polarized monolayer is formed with the apical side on the top and the basolateral side on the bottom of the insert, allowing for assessment of drug permeability and barrier integrity (Figure 1).
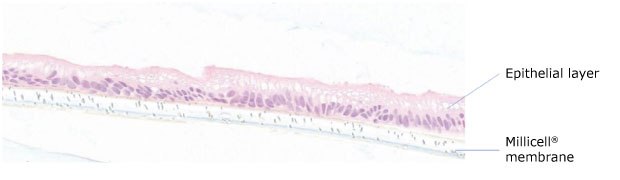
Figure 1.Section of Duo-87 human duodenum patient-derived organoids (PDO) cultured on Millicell® membrane insert for 8 days showing polarized epithelial monolayer. The epithelial layer is at the top of the cross section in pink, and the Millicell® membrane is at the bottom of cross-section in white.
The current standard model for intestinal barrier assays is a colon cancer cell line, Caco2. However, the long growth time (approximately 20 days) and colon characteristics are disadvantageous for accurate assessment of drug effects on the major organ of absorption, the small intestine.
Using the 2.5D system, researchers can monitor over a longer time course and observe of side effects such as cumulative drug toxicity that may be missed with short-term assays. For example, the drug prostacyclin has a very short half-life in solution, making it difficult to ascertain long-term effects. Here we demonstrate 2.5D culture using the small intestine, organoid derived 3dGRO® duodenum organoids (Duo-87) to monitor long-term drug side effects and toxicity.
Growing Small Intestinal Monolayers on Millicell® Inserts
Materials and Methods
- 3dGRO® Duodenum Organoids Duo-87 (SCC322)
- 3dGRO® L-WRN Conditioned Media Supplement (SCM105)
- TrypLE Express
- Matrigel®
- Millicell® 24-well hanging inserts with clear PET membrane
- Millicell® 96 well plate, 1 µm pore size, clear PET membrane (PSRP004)
- Multiwell plates
- Millicell® ERS 3.0 Digital Voltohmmeter (MERS03000)
- Millicell® ERS 3.0 96-well Electrode (MERS0396P)
Single-cell dissociate the patient-derived organoid (PDO) small intestine model Duo-87 on day 5 of culture in complete L-WRN medium using TrpLE Express cell dissociation reagent. Once dissociated, culture Duo-87 cells on 2% GFR Matrigel®-coated Millicell® 24-well hanging cell culture inserts in multiwell plates (Workflow 1). Measure the trans-epithelial electrical resistance (TEER) using a Millicell® ERS 3.0 voltohmmeter.
Workflow
Expand for more details.
Step 2
~10 x 10 µL domes yield 100k cells
Step 3
200k/insert required ~20 x 10 µL domes
Step 4
Need ~240 x 10 µL domes for 24 inserts
Workflow 1. Approximate number of SCC322 Duo-87 organoids and single cells required for seeding a 24-well insert plate.
Duodenum Organoids Drug Toxicity Results
Organoid-derived monolayers cultured in proliferative medium consist largely of epithelial cells derived from the stem cell population. Many of the specialized cells of the small intestine, such as anti-microbial Paneth cells, mucin-producing Goblet cells, and enteroendocrine cells which secrete a variety of hormones, are under-represented.
Culturing the intestinal monolayers in differentiation medium promotes formation of these specialized cells providing a more physiologically reflective model of the gut. The difference in properties between monolayers maintained in either the proliferative or differentiation medium can be seen via TEER values over time (Figure 2).

Figure 2.TEER values for Duo-87 organoids cultured on 1.0 µm pore size 96-well Millicell® plates. TEER values increase for wells switched from proliferative (L-WRN, pink) medium to differentiation (ENR, blue) medium. The L-WRN-grown Duo-87 organoids TEER values do not peak over time, while the ENR-grown Duo-87 organoids TEER values peak at day 10 and gradually decrease back down to the starting value on day 12.
Single cells from duodenum PDOs were seeded into 24-well Millicell® inserts and achieved confluent monolayers by day 10. TEER values were measured daily to produce a time course to assess health of the monolayer until it naturally dissociates after 25-28 days in culture. After 11 days TEER values dropped off for prostacyclin-treated Duo-87 organoids. This is suggestive of a cumulative effect of the drug on membrane integrity (Figure 3).
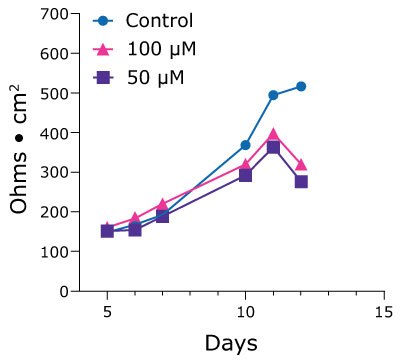
Figure 3.TEER values for Duo-87 organoids on 1.0 µm pore size Millicell® 24-well inserts treated with a control (blue, circles) solution, 100 µM prostacyclin (pink, triangles), or 50 µM prostacyclin (purple, squares). The TEER values for control-treated Duo-87 organoids gradually increase over the time course. The TEER values for the 100 µM and 50 µM prostacyclin-treated Duo-87 organoids increase until day 11, after which the lines steeply drop off.
2.5D cell culture can also help researchers studying drug toxicity reveal more subtle effects that are masked by end-point cell viability assays. Poorly tolerated by the gut, the chemotherapeutic drug afatinib is highly potent but results in a 96% incidence of diarrheagenic side effects. Treating duodenum monolayers with 0.5 µM afatinib in vitro results in cell death and monolayer breakdown. However, lower concentrations demonstrate a dose-dependent lag in the increase of TEER values over time (Figure 4).
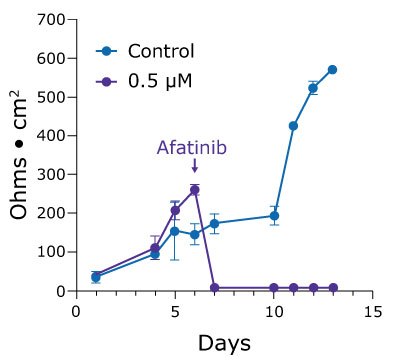
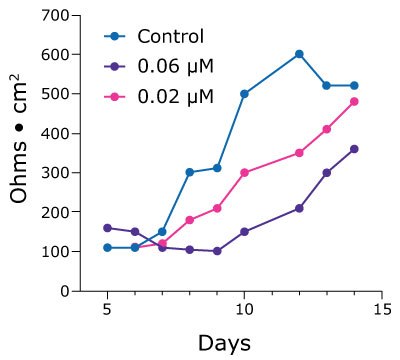
Figure 4. TEER values for Duo-87 organoids on 1.0 µm pore size Millicell® 24-well inserts treated with afatinib. A.) Duo-87 TEER values when treated with a control solution (blue) or with 0.5 µM afatinib (purple). At this concentration, the TEER values gradually increase and then sharply drop off, indicating cell death. B). Duo-87 TEER values when treated with a control solution (blue), 0.06 µM afatinib (purple), or 0.02 µM afatinib (pink). Increasing doses of afatinib below the IC50 value of 0.5 µM result in a dose-dependent lag in TEER value increase.
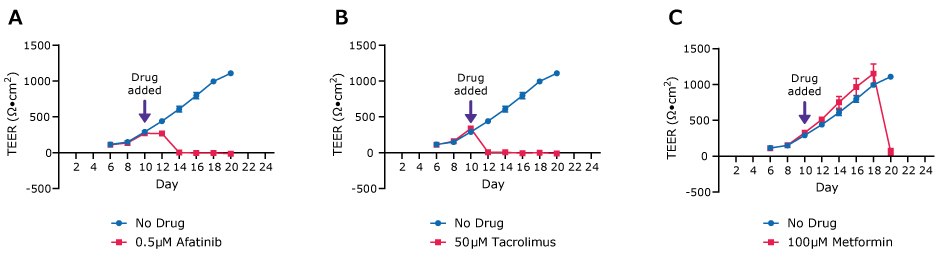
Figure 5.TEER values for Duo-87 organoids on 0.4 µm pore size Millicell® 24-well inserts treated with 0.5 µM Afatinib (SML3109), 50 µM Tacrolimus (PHR1809) and 100 µM Metformin (PHR1084). Compared with the gradually increased TEER value of control sample (blue curve), the drop off of TEER values in drug treated samples (red curves) indicates the cell death. Each data point represents the mean of TEER values from three samples. The error bars represent the standard deviation.
如要继续阅读,请登录或创建帐户。
暂无帐户?