Co-culture of Tumor and Stromal Cells in TrueGel3D™ Hydrogels
Introduction
Tumor microenvironment (TME) is a complex aggregation of malignant cells and non-transformed cells (fibroblasts, neuroendocrine cells, blood and inflammatory cells). The cells within TME have dynamic and tumor regulating functions at all stages of cancer (initiation, progression and metastasis).
Three-dimensional (3D) cell culture systems provide physiologically relevant information in research and drug development more than the traditional 2D culture systems. The additional dimensionality of 3D cultures accurately mimic microenvironments and demonstrate similar cellular responses to in-vivo conditions. TrueGel3D™ hydrogel systems are biochemically defined and easy to use model systems to design biomimetic hydrogels that can mimic natural extracellular matrices (ECM). The objective of this study is to investigate the compatibility of TrueGel3D™ hydrogels for the co-culture of cancer cells and stromal cells.
Methods
TrueGel3D™ hydrogel preparation
All steps were performed in a sterile hood and the volume ratio of each component was added as indicated below.
- Cell suspension was prepared using the following cell culture medium: DMEM (low glucose) containing 4 mmol/L L-Glutamine and DMEM/HAM’sF12 (1:1) containing 2.5 mmol/L L-glutamine. The two media were mixed at a ratio of 1:2 supplemented with 10% (v/v) FBS.
- Water, 10X buffer (pH 5.5) and FAST-DEXTRAN were mixed in a reaction tube.
- TrueGel3D™ RGD integrin adhesion peptide was added to the reaction tube containing FAST-DEXTRAN and mixed immediately to ensure homogenous distribution; it was followed by 5 min incubation to allow attachment of the RGD peptide to the maleimide groups of FAST-DEXTRAN polymer.
- 4.5 µL of CD cell-degradable crosslinker was pipetted and spotted on the surface of a sterile 8-chamber slide with glass bottom chamber slide compatible with inverse microscopy.
- The cell suspension was transferred to the reaction tube containing the FAST-DEXTRAN polymer (cell suspension mix).
- 25.5 µL of cell suspension mix was transferred to the culture dish containing 4.5 µL of CD cell-degradable crosslinker and mixed quickly followed by a 3 min incubation for gel formation (gel formation starts a few seconds after mixing).
- Once gel has formed, 350-400 µL of cell culture medium was added.
- The culture plate was placed in the incubator. The medium was replaced after 1 hour.
- The medium was changed as required for proper growth of the cells
- Cells in hydrogel were treated on the 14th day to proceed to epifluorescence microscopy.
Chemical cell fixation and epifluorescence microscopy
- TrueGel3D™ hydrogels containing cells were fixed using 4% paraformaldehyde in PBS (with Ca++/Mg++) for 1 hour and washed four times for 5 min in PBS (w/o Ca++/Mg++).
- Hydrogels were incubated with 0.5% (v/v) Triton® X-100 in PBS (w/o Ca++/Mg++) for 10 min and washed three times (10 min each) in PBS (w/o Ca++/Mg++).
- Actin staining: Hydrogels were incubated with 1.7 µg/mL phalloidin-TRITC in PBS (w/o Ca++/Mg++) for 1.5 hour in the dark and washed three times (5 min each) in PBS (w/o Ca++/Mg++).
- Nuclei Staining: Hydrogels were incubated with 17 µmol/L Syto 24 Green® (Invitrogen) for 30 min at room temperature in the dark.
- Hydrogels were washed three times (5 min each) with PBS (w/o Ca++/Mg++) and stored in PBS (w/o Ca++/Mg++) at 4 0C before epifluorescence microscopy analysis.
Results
When cultured alone in TrueGel3D™ hydrogels, MCF-7 breast cancer cell lines formed tumor-like spheroids whereas, the human dermal fibroblasts appeared outstretched like in physiological conditions. Co-culture of both cell types in TrueGel3D™ hydrogel retained the physiological and morphological characteristics even after 14 days, and represented a perfect tumor-stroma model to study tumor microenvironment.
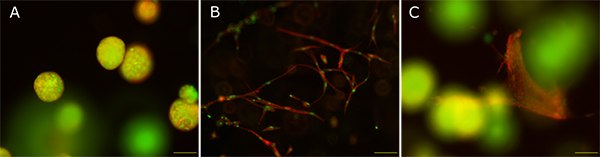
Figure 1. Mono- and co-culture of MCF-7 cells and fibroblasts in TrueGel3D™ hydrogels. A: MCF-7 cells cultured alone, B: human fibroblasts cultured alone, C: Co-culture of MCF-7 and fibroblasts. Red: Actin cytoskeleton; green: nuclei. Scale bar: 100 µM.
Discussion
TrueGel3D™ hydrogel support co-culture models to evaluate the effects of cell-cell interaction and secreted factors on cellular response. MCF7 cell lines grown in TrueGel3D™ hydrogels exhibit characteristics of avascular tumors and allows monitoring of spheroid formation under optical microscopy. Unlike MCF-7 monolayer cells, the spheroids are better models to study drug penetration behavior, cell apoptosis, cell cycle distribution and gene expression1.
The outstretched fibroblasts grown in a 3D environment resemble in-vivo conditions, increase cell migration and proliferative activity of cancer cells, serving better models for epithelial-mesenchymal transition2. The TrueGel3D™ hydrogel also enables post culture analysis (PCR, blotting) to investigate the effects molecular factors on cell behavior.
3D co-culture is not limited to the study of cancer biology3, but instead empirical evidence shows use in various applications including osteogenic differentiation4, chondrocyte differentiation5, angiogenesis6, drug screening7, and peripheral nerve studies8.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?