Single-Use Assemblies
Mobius® 2D and 3D Bag Assemblies
Our Mobius® 2D and 3D assemblies provide efficient and scalable bioprocessing fluid management solutions, from media and buffer preparation to final fill applications. With a full spectrum of standard and custom solutions, at varying levels of certification, you get greater flexibility, mobility, and support for your single-use technologies. Our assemblies and solutions are designed to reduce operator error and process risks while improving efficiency.
- Mobius® Standard 2D Assemblies, are sterilized and ready to use in a wide range of applications.
- Mobius® Standard 3D Drum Assemblies, feature Pureflex™ films and are sterilized and ready to use for fluid storage or processing applications.
- Mobius® Standard 3D Bin Assemblies are made with Ultimus® film or Pureflex™ flms, feature our EZ Fold technique and are designed for easy deployment for transport and storage.
- Mobius® 2D Freeze Assemblies for frozen Liquid Transportation and Storage are made with our PureFlex™ film and Helium integrity tested to assure integrity at temperatures down to -80 °C.
Section Overview
Products
Mobius® Essential Assemblies are standard, off-the-shelf, simple bioprocess bag and tubing assemblies. These plug-and-play assemblies are made of our proven PureFlex™ film and are available in both 2D bags (1–50 L), 3D bags (100–200 L), as well as bioprocess tubing assemblies. These standard assemblies are easily integrated into your current processes providing you with a straight path for scale-up to our Mobius® MyWay assemblies with PureFlex™ film.
- Eliminate design and manufacturing lead time
- 1 L - 200 L sizes that can easily scale up to premium assemblies under Mobius® MyWay program
Need help to find the right Mobius® Essential Assemblies for your processing needs? Fill out a short form to connect with our single-use specialist today.
Mobius® MyWay – Options to Suit Your Supply Needs
Mobius® MyWay is a comprehensive three-tiered program designed to promote a balance of single-use lead times with design flexibility, while enhancing supply security. Mobius® MyWay allows you to have the flexibility of ordering standard and configurable “fit for purpose” assemblies while still receiving the benefits of documentation support and extractable data at reduced lead times. Our unique approach reduces consumable management burden and cost, enhances supply transparency, and improves security.
Mobius® Stock

Mobius® Select

Mobius® Choice

Off-the-Shelf Standard Assemblies
Configure-to-Order Assemblies, Using a Library of Prequalified Components
Highly Customized Solutions
Mobius® Assembly Certification & Release Testing
Mobius® assemblies are available in several levels of certification. Certification of an assembly is based on the qualification of the components in the assembly, the level of leak testing performed during manufacturing, and the testing performed on the assembly lot after manufacturing. The certification level also impacts the shelf life and sterility claims that are made for the assembly.
Helium Integrity Manufacturing Release Test for Increased Integrity Assurance
Mobius® single-use assemblies* can also be tested with a Helium Integrity Manufacturing Release Test. Our Helium Integrity Test is validated to detect defects as small as 2 µm, and is designed to test the entire single-use assembly, including tubing and connection points. Incorporating the Helium Integrity Test into your integrity assurance strategy reduces the risk of leaks or microbial ingress in your manufacturing process.
* Mobius® assemblies for Helium Integrity Test is subject to the design space rules. Please find the details in the Helium Test Data Sheet.
Our Ultimus® film was designed for superior robustness and durability in large-volume processing through a proprietary woven nylon structure. The fluid contact layer is animal origin-free, with low extractable, and supports healthy cell growth as it does not contain Irgafos® 168.
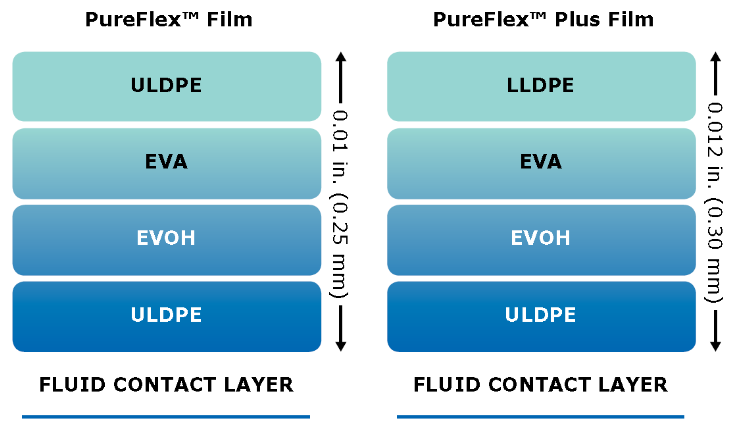
Our PureFlex™ films are used for the construction of Mobius® single-use 2D and 3D bag assemblies. Both PureFlex™ and PureFlex™ Plus films consist of a single fluid contact layer with a low extractable profile, chemically resistant materials, and zero animal-derived components, offering the flexibility and robustness you need throughout your single-use manufacturing processes.
Have a question about Ultimus® film? Contact our technical experts.
Mobius® Single-use Assemblies are Supported by the Emprove® Program – The Smart Way To Master Compliance And Control
Complementing our product portfolio, the Emprove® Program provides convenient access to reliable technical, regulatory and supply information in Emprove® Dossiers to support your risk assessment continuum. Our Advanced Qualification Dossier is customized with specific information for your single-use assembly.
Bioprocess Risk Mitigation
With a global network of sales development specialists and engineers we can support you with system demonstration, design and implementation, at-scale process simulation, and feasibility studies, as well as validation studies and documentation, supported by Millipore® validation services.
Related Resources
- Application Note: Deviron® Detergents for Viral Inactivation
Deviron® C16 and Deviron® 13-S9 Detergents for Solvent/Detergent Viral Inactivation of Plasma in Mobius® Single-Use Containers
- Brochure: Single-use Fluid Management
2D & 3D assemblies, filtration, storage, and transportation solutions
- Application Note: Higher Volume Processing Using Natrix® Q Device
This application note describes a series of tests to assess performance of a Mobius® tubing assembly containing two Natrix® Q Process 600 devices manifolded in parallel.
- White Paper: The Role of BPOG Extractables Data in the Effective Adoption of Single-use Systems
Single-use systems are increasingly popular in biomanufacturing. Their adoption offers numerous advantages for greater efficiency and productivity in applications such as final filtration, mixing and aseptic connections.
- Technical Brief: Shipping Qualification of Mobius® Large Liquid Transportation according to ISTA
Safe and efficient transportation of your large bulk liquid substances is critical. These liquids could include sterile water, buffers, media, intermediates, bulk drug substances and final drug products. Therefore, we completed an extensive ISTA shipping validation to provide the safest transportation for your critical bulk liquids.
- White Paper: Single-Use Upstream Processing: Ultimus® Film Delivers Comparable Cell Growth Performance To Glass
Single-use technologies are increasingly being incorporated into the biomanufacturing workflow to achieve greater efficiency and productivity, reduce capital investment in facilities and equipment, and minimize the risk of cross-contamination.
- Infographic: Prevent Costly Bag Leaks in Single-Use Manufacturing
The Ultimus® Film Difference Designed with a proprietary woven nylon structure, Ultimus® film demonstrates superior strength and resilience to protect against leaks, abrasions, tears and material fatigue.
- Technical Brief: Demonstrated Strength and Durability of Ultimus® Film
Single-use technology is used for different operations throughout biomanufacturing including mixing, storage and transportation.
- Spec Sheet: PureFlex™ Single-Use Process Container Films
PureFlex™ film is used to construct our Mobius® single-use assemblies and Novaseptum® sampling bags. These single-use products are used in biopharmaceutical processes, improving efficiency and productivity while reducing the risk of contamination.
- Spec Sheet: Ultimus® Film
Ultimus® film was designed to meet the needs of more challenging single-use applications such as large-volume liquid processing. Our Ultimus® film technology provides enhanced bag strength, improved durability and leak resistance through a novel strength layer reinforced by woven nylon.
- Data Sheet: Mobius® 2D Freeze Assembly
Mobius® 2D Freeze Assembly Data Sheet
- Spec Sheet: Mobius® Essential Assembly
Mobius® Essential Assembly Spec Sheet
To continue reading please sign in or create an account.
Don't Have An Account?