Leak Resistant Film for Single-Use Bioprocessing Bags
Single-use technology is used throughout biomanufacturing in single-use bioreactors, mixers, as well as storage and transportation solutions. Selecting a single-use bioprocessing film requires careful consideration of specific application needs. The integrity of the film used in single-use containers is critical to minimizing leaks and maintaining reliable manufacturing operations. Ultimus® film is designed to maintain container integrity, even in challenging large volume single-use applications. This article summarizes performance of this film against the American Society for Testing Materials (ASTM) standards, commonly used to assess strength and robustness in bioprocessing films.
Read more about
Structure of Ultimus® Film
Ultimus® film is a multilayered film that provides enhanced bag strength, improved durability and leak resistance through a novel strength layer reinforced by woven nylon. The fluid contact layer is made of ultra-low density polyethylene (ULDPE), promotes healthy cell growth, and is free of Irgafos® 168 and animal origin-free components. The strength layer contains woven nylon, sandwiched between layers of ethylene vinyl acetate (EVA) and provides both strength and flexibility. The gas barrier layer is made of ethylene vinyl alcohol copolymer (EVOH) while the film’s low-density polyethylene (LDPE) outer layer minimizes leaks through resistance to abrasion, puncture, stretching and tearing.
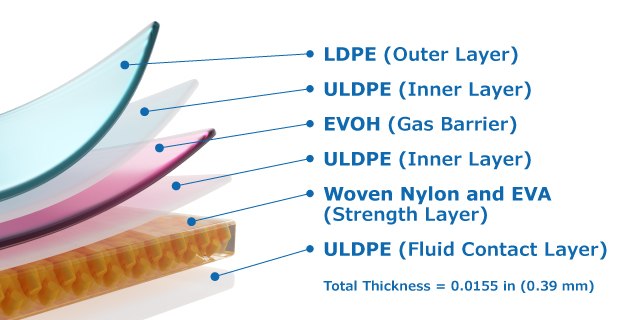
Figure 1.Schematic of Ultimus® film structure showing the LDPE outer layer, EVOH gas barrier, Woven nylon and EVA strength layer and ULDPE safe fluid contact inner layer. The film thickness is 0.0155 in. (0.39 mm).
Evaluating Ultimus® Film Strength Against ASTM Standards
The ASTM defines technical standards for various materials, products and systems, including film. ASTM standard test methods were used to compare Ultimus® film performance to the average performance of five commercially available bioprocessing films.
Tensile Strength (ASTM D882: Tensile Properties of Thin Plastic Sheeting)
Results show that Ultimus® film has 2.8x higher tensile strength compared to the average of the five commercially available single-use bioprocessing films tested.
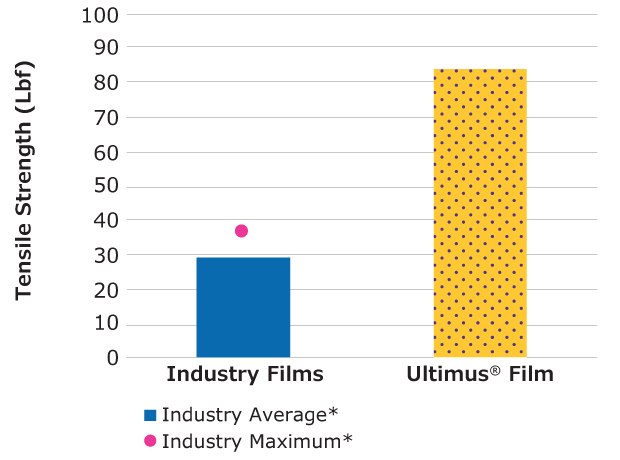
Figure 2.Relative tensile strength of Ultimus® film compared to other single-use films.
Abrasion Resistance (ASTM F3300: Abrasion Resistance of Flexible Packaging Films using a Reciprocating Weighted Stylus)
Ultimus® film showed 10x higher abrasion resistance compared to the average of five industry films tested.
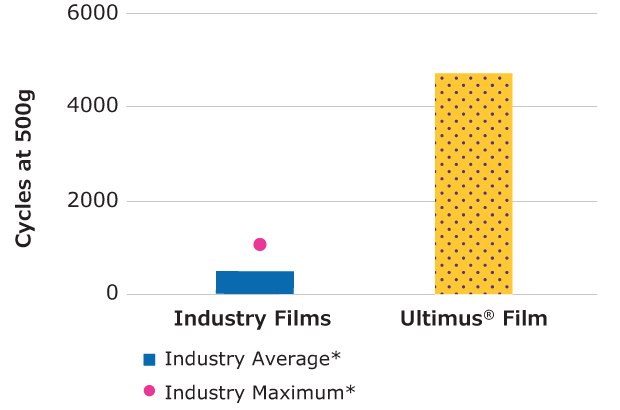
Figure 3.Relative abrasian resistance of Ultimus® film compared to other single-use films.
Puncture Force (ASTM F1306: Slow Rate Penetration Resistance of Flexible Barrier Films and Laminates)
The puncture force for Ultimus® film was 2x higher compared to the average of five commercially available single-use bioprocessing films tested.
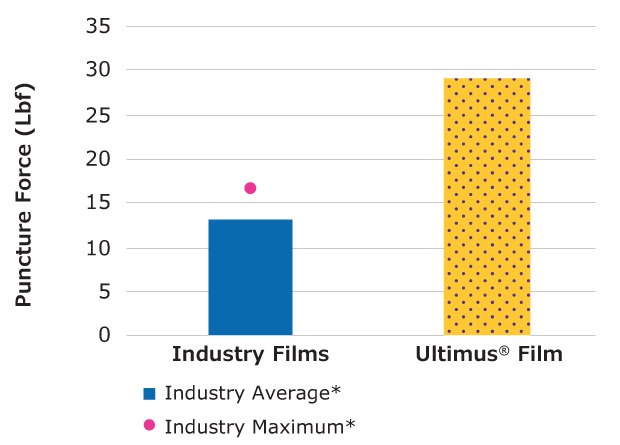
Figure 4.Relative force required to puncture Ultimus® film compared to other single-use films.
Number of Pinholes (ASTM F392: Standard Practice for Conditioning Flexible Barrier Materials for Flex Durability)
Ultimus® film contained 100% fewer pinholes compared to the average of five commercially available single-use bioprocessing films tested.
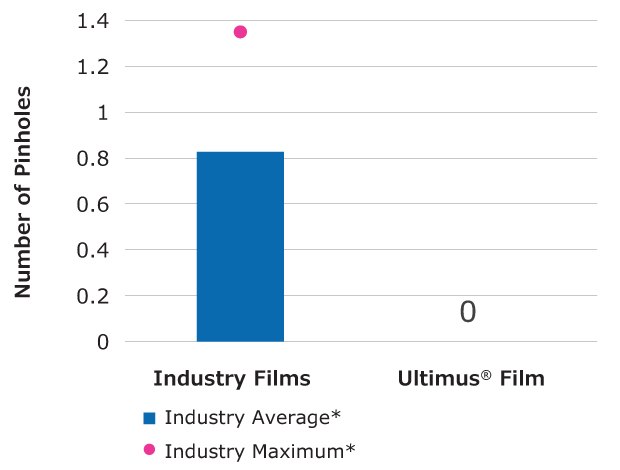
Figure 5.Relative flex durability of Ultimus® film compared to other single-use films.
Structure and Durability Testing of Containers at Large Scale
Both the damage resistance of the single-use film structure and seal strength are important in bioprocessing applications. The following tests assessed structure and durability of containers made with Ultimus® film.
Burst Container for Structure Evaluation
- Test description: This test evaluates material structure by measuring the pressure a bag can withstand before bursting. Mobius® single-use bags (10 in. X 8 in.) made with Ultimus® and PureFlex™ films were filled with water and pressurized until they burst.
- Results: Single-use bags made with Ultimus® film withstood 27 psi of pressure, a 2.8x improvement in bag assembly structure and seal strength over bags made with PureFlex™ film.
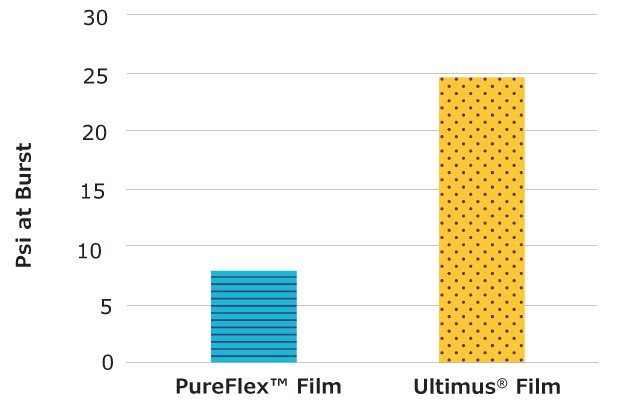
Figure 6.Bags made from Ultimus® film required 2.8x more pressure to burst compared to bags made with PureFlex™ film.
Large-Scale Multiple Fill and Drain for Robustness Testing
- Test description: In large-volume applications, extreme overhandling of the bags can contribute to leaks. To assess robustness under extreme conditions, Mobius® bag assemblies made with Ultimus® film were gamma irradiated at 25-50 kGy, filled to 1000 L, held for an hour, checked for leaks, and then drained; this process was repeated 6 times on three separate bags.
- Results: No leaks were observed in any bag after repeated filling and draining, confirming the robustness of 1000 L bags made with Ultimus® film.
Practical Implications of Superior Strength and Robustness
Testing against ASTM standards demonstrated the improved strength and robustness of Ultimus® film as compared to other commercially available single-use films. In addition, performance of Mobius® 500 L process containers with Ultimus® film in 500 L Stainless Steel Transport Bin was verified against the ISTA 3E Transport Test, further demonstrating strength and robustness.
This improved strength and robustness results in containers made with Ultimus® film having significantly improved structure and seal strength as compared to containers made from PureFlex™ film. This superior strength was demonstrated under extreme conditions with no leaks observed following irradiation and multiple fill/drain cycles involving 1000 L of liquid.
This resilient film offers opportunities for implementing single-use technology into bioprocessing operations, minimizing the risk of leaks, and maintaining reliable manufacturing operations in both small and large volume applications.
References
To continue reading please sign in or create an account.
Don't Have An Account?