Endotoxin-free Plasmid DNA Purification
Plasmid DNA purification kits that reduce levels of endotoxin typically have lengthy and tedious protocols. The GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit enables rapid isolation of endotoxin-free plasmid DNA. The kit follows a streamlined procedure that can be completed in as fast as 35 minutes. It combines the convenience of a bind, wash and elute format with an optimized binding solution that prevents endotoxins from carrying through to the final product. This allows researchers to consistently achieve levels of endotoxin that are less than 0.1 endotoxin units per µg of plasmid DNA without having to sacrifice high plasmid recoveries.
Products
What are Endotoxins?
Endotoxins, also known as lipopolysaccharides (LPS), are the major component making up the outer membrane of Gram-negative bacteria. Approximately three-quarters of the bacterial surface consist of these molecules.1 The outer membrane provides a barrier that protects the cell from large molecules and hydrophobic compounds yet is permeable to small hydrophilic molecules that are essential for cell survival.2 Endotoxins may also play major roles in host cell interactions, such as colonization and resistance to phagocytosis.2
The basic structure of an endotoxin is similar among Gram-negative bacteria but the chemical composition can vary widely. The structure consists of three distinct regions: lipid A, core oligosaccharide, and O-antigen (Figure 1). Lipid A is the hydrophobic, membrane-anchoring region of the endotoxin and is highly conserved among Gram-negative bacteria.1 It consists of an N-acetylglucosamine dimer (NAG) with 6 to 7 fatty acids attached.2 The core oligosaccharide is attached to the NAG dimer and consists of a short chain of sugars. This region is conserved between members of a particular bacterial genus but will vary among genera.2 The third component of the endotoxin is the O-antigen, which is the hydrophilic portion of the endotoxin that extends out into the cells' environment. The O-antigen is attached to the core oligosaccharide and consists of 15 to 20 repeating subunits of three to five sugars.3 The composition of these sugars is strain dependent and can be used to distinguish different bacterial strains.4
Endotoxins are a common contaminant in nucleic acid preparations, particularly when purifying plasmid DNA. During cell lysis, high concentrations of endotoxins are released into the lysate. Like DNA, endotoxins are negatively charged and co-purify with plasmid DNA in both silica-based and anion-exchange-based systems. Endotoxin monomers will normally vary between 10 and 20 kDa but can form large aggregates greater than 1000 kDa5 resulting in co-purification in size exclusion columns and cesium chloride density gradients. Therefore, both standard and traditional plasmid purification systems can result in high levels of endotoxin contaminating the purified plasmid DNA.
While not every molecular biology technique requires the use of endotoxin-free plasmid DNA, transfection of sensitive mammalian cell lines is a major application where endotoxin-free DNA is essential. Transfection can be defined as the introduction of foreign DNA or RNA into a eukaryotic cell. As practiced, genes are transfected into cells so their regulation and expression can be studied. Data has demonstrated that endotoxins reduce cell viability and transfection efficiencies among different eukaryotic cell lines, such as COS-7 and Jurkat cells.6 Furthermore, certain cell lines appear to be more sensitive to endotoxins than others, such as HuH-7 cells.6 It is known that endotoxins can have complex effects on mammalian systems, such as nonspecific activation of the immune system, stimulation of cytokine overproduction, and induction of toxic-shock syndrome.7 Principally, endotoxins introduce a variable that can influence the outcome and reproducibility of a given transfection experiment.
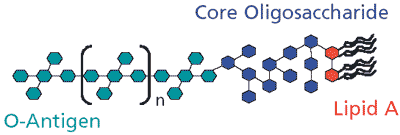
Figure 1.Schematic of bacterial endotoxin (lipopolysaccharide).
Plasmid Purification Method
Culture Preparation
Recombinant E. coli strains DH5α pCMV-SPORT-β-gal (Invitrogen, Carlsbad, CA) and HB101 pBICEP-CMV-1-LacZ (Product No. E0779) were inoculated into LB media containing 100 µg/ml of ampicillin (Product No. A9518). Bacterial cultures were grown, shaking at 275 rpm for 17 hours at 37 °C.
Plasmid Preparation
Each sample was prepared by harvesting 150 ml of bacterial culture by centrifugation at 5,000 x g for 10 minutes. The pellets were thoroughly resuspended in 12 ml of Resuspension Solution and then subjected to a modified alkaline-SDS lysis by the addition of 12 ml of Lysis Solution. The lysates were neutralized with the addition of 12 ml of Neutralization Solution, which resulted in the formation of a white flocculent precipitate containing denatured proteins, lipids, SDS, chromosomal DNA, and other cell debris.
The lysates were transferred to individual filters and incubated at room temperature for 5 minutes. The lysates were drawn through the filters by vacuum resulting in a clarified lysate. The clarified lysates were prepared for loading onto the binding columns by the addition of 9 ml of Binding Solution. Binding columns were prepared by attaching them to a standard laboratory vacuum manifold followed by the addition of 12 ml of Column Preparation Solution, which was drawn through by vacuum. The lysate mixtures from above were loaded onto the binding columns and drawn through by vacuum. Contaminants were removed from the columns by washing with 12 ml of Wash Solution 1, followed by 12 ml of Wash Solution 2. The columns were allowed to dry for 10 minutes while still under vacuum and then transferred to individual collection tubes. Finally, the plasmid DNA was eluted by the addition of 3 ml of endotoxin-free water and centrifugation at 3,000 x g for 5 minutes. The recovered eluates were then ready for analysis and use in downstream applications.
Plasmid Analysis
Plasmid concentrations were determined by taking absorbance readings at 260 nm. Total yield (mg) was calculated by multiplying the concentration by the volume of recovered eluate. The purity of the samples was determined by calculating the ratio of absorbance at 260 nm/280 nm. Samples were run on a 1% TBE agarose gel at 100 volts and visually inspected to confirm their yield and purity.
Endotoxin Testing
Endotoxin levels were determined by using the QCL-1000 Quantitative Chromogenic LAL Kit (BioWhittaker, Walkersville, MD) following the Test Tube Method.
Transient transfection and measurement of reporter gene activity
Human hepatoma cell line (HuH-7; provided by Dr. Clifford J. Steer, University of Minnesota Medical School) was cultured in Dulbecco's Modified Eagle's Medium (D-MEM). The medium was supplemented with 10% fetal bovine serum (FBS) and 4 mM L-glutamine. For the purpose of transfection, HuH-7 cells were seeded at approximately 1.0-1.5 x 105 cells per well in 2 ml of growth media onto 6-well tissue culture plates (Corning Inc., Corning, NY). The plates were maintained in a humidified CO2 incubator at 37 °C until the cells were 60-70% confluent, which usually took 24-30 hours.
The cells were transfected with ESCORT II™ Transfection Reagent (Product No. L6037), according to the product's information protocol unless otherwise stated. To summarize for one well of a 6-well culture plate, 15 µL of hydrated ESCORT II reagent was diluted with 60 µL of serum-free D-MEM in a sterile microfuge tube. Next, 3 µg of purified plasmid DNA was diluted with 75 µL of DNA diluent (Product No. D6813). This mixture was incubated at room temperature for 1 to 5 minutes.
The diluted DNA sample was then added to the ESCORT II/ serum-free D-MEM mixture and incubated at room temperature for 5 to 10 minutes. During this incubation, the HuH-7 cells were gently washed twice with 2 ml of serum-free D-MEM before adding 850 µL of serum-free D-MEM to each well. The ESCORT II/DNA complex was then added directly to the HuH-7 cells. The cells were incubated in a tissue culture CO2 incubator at 37 °C for 4 to 5 hours after which time 1 ml of D-MEM supplemented with 20% FBS and 4 mM L-glutamine were added. As a negative control, HuH-7 cells were treated with the same transfection mixture without plasmid DNA.
The efficiency of transfection was determined by analyzing the HuH-7 cell extracts for gene activity 72 hours after beginning the transfection. This activity was measured by using the β-Galactosidase Reporter Gene Activity Detection Kit (Product No. GAL-A) following the kit's product information protocol. Transfection efficiency was reported as β-galactosidase units per plate, which represents an average of six independent transfection replicates for each sample.
Plasmid Purification Results and Discussion
The GenElute™ HP Endotoxin-Free Maxiprep Kit was evaluated by purifying two different plasmids from two different E. coli strains. Following purification, the plasmids were analyzed for recovery and purity. The calculated yield for plasmids pCMV-SPORT-β-gal and pBICEP-CMV-1-LacZ were very high; 1.4 mg of plasmid DNA recovered from a 150-ml LB culture for both. Each sample was also found to be of high purity, indicated by an absorbance ratio at 260 nm/280 nm of 1.9. Ratios between 1.8 and 2.0 typically indicate plasmid preparations of high quality. The purity was also confirmed by agarose gel electrophoresis, which showed no evidence of genomic DNA or RNA contamination (data not shown).
For comparison purposes, three other commercially available kits were used to prepare plasmids from the same bacterial cultures used to prepare the samples described above. The first kit utilizes a non-endotoxin-free silica-based system, the second is an endotoxin-free kit that utilizes anion-exchange technology, and the third is an endotoxin-reducing silica-based kit that incorporates magnetic particles into the purification process. All samples were prepared by following the manufacturer's protocols. In addition, plasmid pCMV-SPORT-β-gal was prepared from a separate culture by purifying on two separate cesium chloride gradients (2X CsCl banding). While not considered to be an endotoxin-free method, this has long been considered the standard for preparing highly purified plasmid DNA. The clarified lysate for the CsCl sample was prepared identical to the samples purified using the GenElute™ HP Endotoxin-Free Maxiprep Kit. The plasmid DNA was then precipitated by the addition of sodium acetate and isopropanol, followed by centrifugation. The plasmid DNA pellet was dried and then resuspended in TE buffer, pH 8.0. The protocol of Sambrook and Russell8 was followed to prepare the cesium chloride gradients and to further purify the plasmid DNA.
Plasmid yields for all the preparations were calculated from absorbance readings taken at 260 nm. The results were reported in milligrams (mg) and compared in Tables 1 and 2. Plasmid yields obtained from the GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit were similar or better than the competition. Next, endotoxin levels were determined using the Chromogenic LAL kit from BioWhittaker. The results were reported as Endotoxin Units per microgram of plasmid and compared in Tables 1 and 2.
The GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit showed a significant reduction in endotoxins when compared to a similar silica-based kit. Endotoxin levels were also among the lowest when compared to the other available endotoxin-free kits, as well as the 2X CsCl sample. Furthermore, the amount of time per preparation was compared in Tables 1 and 2; this revealed the most significant difference between the endotoxin-free kits. The GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit took only 35 minutes to complete, while the two other competing endotoxin-free kits took over four times longer. Only the other silica-based system was comparable in preparation time, but the purified sample had higher levels of endotoxin.
To further evaluate the plasmid purified using GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit, a sample from each preparation was transfected into HuH-7 cells, a human hepatoma cell line. HuH-7 cells were chosen for this experiment because they have been reported to be more sensitive to endotoxins during transfection than other commonly used cell lines.6 Transfections were also performed with the samples prepared from the other commercially available kits, as well as with the 2X CsCl sample. Plasmids pCMV-SPORT-β-gal and pBICEP-CMV-1- LacZ both contain the β-lactamase reporter gene and can be detected using a β-galactosidase detection kit following transfection.
The transfection efficiency data for the pCMV-SPORT-β-gal samples are shown in Figure 2. The results show that the sample purified using the GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit had the highest transfection efficiency when compared to the other commercially available kits. The HP Endotoxin-Free Maxiprep sample also outperformed the 2X CsCl sample, which was once considered the standard for purity. The transfection efficiency data for the pBICEP-CMV-1-LacZ samples are shown in Figure 3. The patterns of efficiencies were almost identical to that seen with the pCMV-SPORT-β-gal samples. The transfection efficiency for the GenElute HP Endotoxin-Free Plasmid Maxiprep Kit was the highest, even outperforming the anion-exchange kit that had slightly lower endotoxin levels. This result suggests the possibility of other contaminates besides endotoxins that may influence the efficiency of transfection. These transfection experiments demonstrate that plasmid purified using the GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit is of high quality and will outperform the competition as well as traditional methods such as 2X CsCl banding.
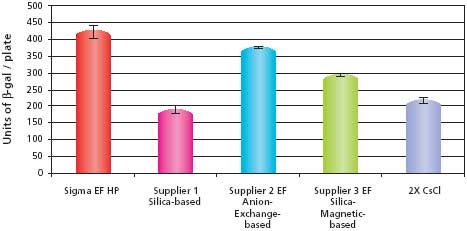
Figure 2.Comparison of transfection efficiencies into HuH-7 cells using different purification systems with pCMV-SPORT-β-gal. The data shows the average and standard deviations of six replicates from each sample prepared. Untransfected HuH-7 cells reported no β-galactosidase activity (data not shown). Details described in Materials and Methods section.
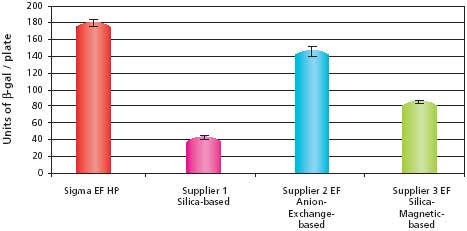
Figure 3.Comparison of transfection efficiencies into HuH-7 cells using different purification systems with pBICEP-CMV-1-LacZ. The data shows the average and standard deviations of six replicates from each sample prepared. Untransfected HuH-7 cells reported no β-galactosidase activity (data not shown). Details described in Materials and Methods section.
The GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit is a unique system that delivers high plasmid recoveries with levels of endotoxin that are consistently below the industry standard of 0.1 EU/µg. The kit’s innovative technology combined with a straightforward procedure enables the user to complete the process in 35 minutes, making it the fastest endotoxin-free kit available. The GenElute™ HP Endotoxin-Free Maxiprep Kit outperforms both current and traditional purification methods, delivering high quality plasmid DNA necessary for high efficiency transfection of sensitive eukaryotic cell lines.
References
To continue reading please sign in or create an account.
Don't Have An Account?