L-Cysteine in Cell Culture
What is L-Cysteine?
L-cysteine and its oxidation product, L-cystine, are essential amino acids and common media supplements in cell culture. These supplements are often used in biomanufacturing, tissue engineering, and specialty cell culture, including hybridoma and Chinese Hamster Ovary (CHO) culture. Virtually all classical and serum-free media contain some form of L-cysteine. We offer L-cystine supplements for cell culture from animal and non-animal sources:
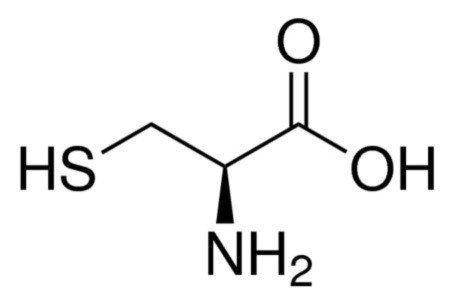
Figure 1:L-cysteine chemical structure
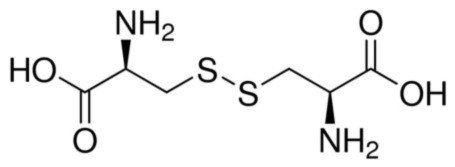
Figure 2:L-cystine chemical structure
L-Cysteine in Cell Culture Media
L-cysteine is oxidized to L-cystine relatively easily in vitro. The performance and stability of cell culture media is affected by the ratios of its various forms. Supplementing media for serum-free culture is also complicated by the R-chain(s). These R chains contain sulfur atoms that participate in sulfur oxidation, reduction and radical chemistry, and metal coordination chemistry.
Copper in media may affect the solubility of these amino acids, forming complexes that cannot be absorbed by cells. Cuprous copper may also reduce cystine to cysteine.
L-cysteine is formulated into F-12 Coon's Modification, L-15 Medium, McCoy's 5A Modified Medium, MCDB Medium, Ham’s F-10 Nutrient Mixtures, Ham’s F-12 Nutrient Mixtures, Ham's F-12 Nutrient Mixture Kaighn's Modification (F12K), Serum-Free/Protein Free Hybridoma Medium. When these media are stored as liquids, L-cysteine can be converted to L-cystine. Under extreme conditions, L-cystine can be further oxidized.
L-cystine is formulated directly into Ames' Medium, Basal Medium Eagle (BME), Click's Medium, Dulbecco's Modified Eagle's Medium (DMEM), Glascow Modified Eagle's Medium (GMEM), Iscove's Modified Dulbecco's Medium (IMDM), Minimum Essential Medium Eagle (EMEM), RPMI-1640, and other media.
Additionally, various ratios are formulated into DMEM/Ham's Nutrient Mixture F-12 (50:50), Medium 199, NCTC Medium, Waymouth Medium MB, Williams Medium E. Media used for biomanufacturing and tissue engineering frequently contain additional L-cysteine equivalents, such as N-acetylcysteine.
L-Cysteine vs L-Cystine
Cysteine has the empirical formula C3H7O2NS and a molecular weight of 121.16. Cystine is composed of two cysteine molecules joined by a disulfide bond. It has the empirical formula C6H12O4N2S2 and a molecular weight of 240.31. In cells, both molecules contain sulfur atoms that provide sites for redox activity and electron transfer.
Cysteine and cystine enter cells by different transporters. This helps specific cells control how they utilize L-cysteine in its various forms. These forms could be L-cysteine, L-cystine, N-acetyl cysteine (NAC), mixed disulfides, or various peptides. In addition to its role in protein synthesis, cysteine is the functional activity of the tri-peptide glutathione.
Cysteine also serves a very important, though indirect, role of protecting cells from oxidative stress. It is the rate-limiting amino acid used in the synthesis of the tri-peptide glutathione. Glutathione is the primary aqueous antioxidant involved in blocking lipid peroxidation and dehydroascorbic acid oxidation. In the absence of L-cysteine or its equivalents, glutathione is rapidly depleted and cells will die
Primary Functions of L-Cysteine and L-Cystine in Cells
Cystine and cysteine are nutritionally considered to be non-essential amino acids because they are synthesized in the livers of mammals. However, for cell culture applications they are essential amino acids that must be supplied from an exogenous source. Creating an effective media for your cells is complicated because these two amino acids are non-enzymatically inter-convertible and unstable.
Cystine residues are formed by the oxidation of cysteine sulfhydryls in proteins. The formation and disruption of the inter- and intra- protein disulfide bonds play important roles in determining secondary and tertiary structures. Therefore, this oxidation can affect the structure of the protein within the cell.
Many cells can also metabolize cysteine to form hypotaurine and taurine. These two molecules help detoxify cell culture media by reacting with hydroxyl free radicals and hypochlorous acid, respectively.
Cysteine can also be involved in protein synthesis, auto-oxidation, synthesis of glutathione, and synthesis of Enzyme CoA. Understanding their chemistry and the ways that cells utilize them are critical factors for designing a successful culture medium.
Cysteine and Cystine Radical Chemistry
In vitro, cysteine is freely soluble and exists initially as a neutral amino acid. It is unstable and undergoes non-enzymatic autoxidation, which can be accelerated by ferric and/or cupric cations. These reduced cations complex with the amino acid, catalyzing the conversion of hydrogen peroxide into hydroxyl free and sulfide free radicals.
Cysteine oxidation is greatly affected by the transition metals copper and iron. Univalent cysteine oxidation generates a thiyl radical. This radical can participate in reactions with di-molecular oxygen, another cysteine thiyl radical (forming cystine), or other free radicals, propagating the oxidative process.
Free radical propagation is terminated when the cysteine thiyl reacts with another thiyl free radical. Cystine is susceptible to further oxidation to form cysteic acid and other oxidative products. The cystine radical can also react with hydrogen peroxide to form the hydroxyl free radical.
The described reactions can make cystine and cysteine unavailable for cell use or make a cell culture medium toxic. It is important to understand their ratios and amounts in liquid media that your cells need. Cell types vary in their ability to uptake of both amino acids.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?