Blood Card - Extraction & Amplification WGA Protocol
Blood cards provide the convenience of archiving small volumes of blood. However, many times genomic DNA from these samples is limited, which may hinder the researcher’s ability to perform downstream analysis. This protocol provides a simple and convenient method to extract genomic DNA from a blood card. Once the DNA has been extracted, it can then be amplified using the amplification protocol
DNA Extraction
We recomment the GenElute™ Blood Genomic DNA Kit (NA2010) for this procedure.
1. Cut a disc from a dried blood card (200 μL spotted) into several 2 mm by 2 mm pieces and place the pieces into a 1.5 mL microcentrifuge tube.
2. Add 40 μL Proteinase K and 1.0 mL Resuspension Solution.
3. Add 300 μL of Lysis Solution C and vortex thoroughly for 15 seconds.
4. Incubate at 55 °C for 10 minutes.
5. After the incubation, transfer the liquid (discard blood card remaining in the microcentrifuge tube) to a 15 mL conical tube.
6. Add 500 μL of Column Preparation Solution to the GenElute Miniprep Binding Column and centrifuge at 12,000 × g for 1 minute.
7. Discard the flow-through liquid.
Note: The Column Preparation Solution maximizes binding of DNA to the membrane resulting in more consistent yields.
8. Add 900 μL of 95–100% ethanol to the lysate in the 15 mL conical tube and mix thoroughly by vortexing 5–10 seconds.
9. Transfer the contents of the tube into the treated column from step 6. Centrifuge at ≥6500 × g for 1 minute. Repeat until all of the lysate has been passed through the column
10. Discard the collection tube and flow-through. Place the column in a new 2 mL collection tube.
11. Add 500 μL of Prewash Solution (be sure to dilute with ethanol prior to first use) and centrifuge for 1 minute at ≥6500 × g.
12. Discard the collection tube containing the flow-through and place the binding column into a new 2 mL collection tube.
13. Add 500 μL of Wash Solution (be sure to dilute with ethanol prior to first use) to the binding column and centrifuge at maximum speed (12,000–16,000 × g) for 3 minutes to dry the binding
column.
14. Pipette 400 μL of Elution Solution onto the column and centrifuge for 1 minute at ≥6500 × g to elute the DNA.
15. Store the eluted DNA at –20 °C or proceed to the amplification step
WGA Amplification
Protocol for GenomePlex™ Whole Genome Amplification performed with GenomePlex™ Whole Genome Amplification Kit (WGA1) and/or Complete Whole Genome Amplification Kit (WGA2).
Note: If using WGA2 there is no need to supply DNA polymerase as the enzyme is provided with the kit
Fragmentation
Prepare DNA solution of 1 ng/µL from whole blood extraction protocol described above.
Add 1 µL of 10X Fragmentation Buffer to 10 µl DNA (1 ng/µL) in a PCR tube.
Place the tube in a thermal cycler at 95 °C for exactly 4 minutes. Note, the incubation is time sensitive and any deviation may alter results.
Immediately cool the sample on ice and centrifuge briefly.
Library Preparation
Add 2 µL of 1x Library Preparation Buffer.
Add 1 µL of Library Stabilization Solution.
Mix thoroughly and place in thermal cycler at 95 °C for 2 minutes.
Cool the sample on ice and centrifuge briefly.
Add 1 µL Library Preparation Enzyme, mix thoroughly, and centrifuge briefly.
Place sample in thermal cycler and incubate as follows:
16 °C for 20 minutes
24 °C for 20 minutes
37 °C for 20 minutes
75 °C for 5 minutes
4 °C hold
Remove samples from thermal cycler and centrifuge briefly. Samples may be amplified immediately or stored at - 20 °C up to three days.
Amplification
Add the following reagents to the 15 µL reaction from the previous step:
7.5 µL 10x Amplification Master Mix
47.5 µL Nuclease Free Water
5.0 µL JumpStart™ Taq DNA Polymerase (12.5 units) for WGA1
-or-
5.0 µL WGA DNA Polymerase for WGA2
Mix thoroughly, centrifuge briefly, and begin thermocycling:
Initial Denaturation: 95 °C for 3 minutes
Perform 14 cycles as follows:
Denature: 95 °C for 15 seconds
Anneal/Extend: 65 °C for 5 minutes
After cycling is complete, maintain the reactions at 4 °C or store at –20 °C until ready for analysis or purification.
Reamplification
1. Add 10 µL of 1 ng/µL WGA amplified DNA to a PCR tube or multiwell plate.
Note: It is necessary to clean up the WGA reaction to decrease possible bias in the reamplification. We recommend using the GenElute™ PCR Clean-Up Kit (Product Number NA1020) or standard purification methods that isolate single and double stranded DNA.
2. Create amplification mix. For each reamplification reaction, add the following to the WGA amplified DNA (step 1):
47.5 µL of Nuclease-Free Water
7.5 µL of 10X Amplification Master Mix
5 µL of WGA DNA Polymerase
3. Vortex thoroughly, centrifuge briefly, and begin thermocycling. The following profile has been optimized for a PE 9700 or equivalent thermocycler:
Initial Denaturation 95 °C for 3 minutes Perform 14 cycles as follows:
Denature 94 °C for 15 seconds
Anneal/Extend 65 °C for 5 minutes
After cycling is complete, maintain the reactions at 4 °C or store at –20 °C until ready for analysis or purification. The stability of WGA DNA is equivalent to genomic DNA stored under the same conditions.
Purification
Purification of amplified products with the GenElute™ PCR Clean-Up Kit (NA1020)
1. Insert a GenElute™ Miniprep Binding Column (with a blue O-ring) into a provided collection tube, if not already assembled. Add 0.5 mL of the Column Preparation Solution to each miniprep column and centrifuge at 12,000 x g for 30 seconds to 1 minute. Discard the eluate.
Note: The Column Preparation Solution maximizes binding of the DNA to the membrane resulting in more consistent yields.
2. Add 5 volumes of Binding Solution to 1 volume of the PCR reaction and mix. For example, add 500 µL of Binding Solution to 100 µL of the PCR reaction. Transfer the solution into the binding column. Centrifuge the column at maximum speed (12,000 to 16,000 x g) for 1 minute. Discard the eluate, but retain the collection tube.
3. Replace the binding column into the collection tube. Apply 0.5 mL of diluted Wash Solution to the column and centrifuge at maximum speed for 1 minute. Discard the eluate, but retain the collection tube.
Note: Be sure to add ethanol to the Wash Solution Concentrate prior to first time use. See Preparation Instructions.
4. Replace the column into the collection tube. Centrifuge the column at maximum speed for 2 minutes, without any additional wash solution, to remove excess ethanol. Discard any residual eluate as well as the collection tube.
5. Transfer the column to a fresh 2 mL collection tube. Apply 50 µL of Elution Solution or water to the center of each column. Incubate at room temperature for 1 minute.
Note: When eluting with water, make sure that the pH of the water is between 5.5 and 8.5. Elution may also be performed using the Elution Solution diluted 10-fold with water.
6. To elute the DNA, centrifuge the column at maximum speed for 1 minute. The PCR amplification product is now present in the eluate and is ready for immediate use or storage at –20 °C.
Quantification of Amplified Products
The amount of DNA amplified using GenomePlex™ Whole Genome Amplification can be detected with or without purification. For the highest quality samples of DNA, it is strongly recommended to purify the samples after amplification. The amplified products can be measured with the PicoGreen® dsDNA Quantitation Assay from Molecular Probes Inc. (#P-7589). Another method of detecting the amplified products is spectrophotometric absorption (OD260) on a NanoDrop® spectrophotometer. This instrument can measure absorbance on 1 μL of sample over a large dynamic range, from 2–3700 ng/μL.
Application Data
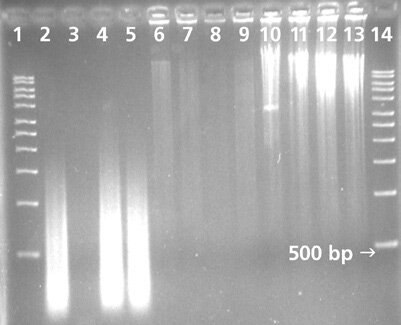
Figure 1.Application Data
Products were amplified using the GenomePlex® Whole Genome Amplification Kit (Cat. No.WGA1), Supplier A’s kit and Supplier Q’s kit. Products were resolved on a 1.5% agarose gel. 5 μL of amplified product was added to each well.
Lane 1 – 1kb Marker
Lane 2 – GenomePlex® Positive Control
Lane 3 – GenomePlex® Negative Control
Lane 4 – Our Blood Card
Lane 5 – Our Blood Card
Lane 6 – Competitor A Positive Control
Lane 7 – Competitor A Negative Control
Lane 8 – Competitor A Blood Card
Lane 9 – Competitor A Blood Card
Lane 10 – Competitor Q Positive Control
Lane 11 – Competitor Q Negative Control
Lane 12 – Competitor Q Blood Card
Lane 13 – Competitor Q Blood Card
Lane 14 – 1kb Marker
Required Products
NA2010
E7023
W4502
WGA1
WGA2
D9307
NA1020
Materials to be Supplied by the User
Blood card
1.5 mL microcentrifuge tubes
Microcentrifuge (with rotor for 2 mL tubes)
55 °C water bath or heat block
GenElute and JumpStart are trademarks of Merck KGaA, Darmstadt, Germany and/or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources
Materials
To continue reading please sign in or create an account.
Don't Have An Account?