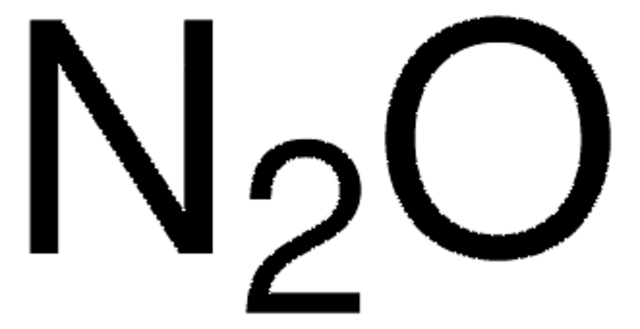00583
Nitrous oxide
≥99.998%
Synonym(s):
Dinitrogen monoxide, Laughing gas
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
N2O
CAS Number:
Molecular Weight:
44.01
Beilstein:
8137358
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
1.53 (15 °C, vs air)
vapor pressure
51.7 mmHg ( 21 °C)
Assay
≥99.998%
bp
−88 °C (lit.)
mp
−91 °C (lit.)
SMILES string
[O-][N+]#N
InChI
1S/N2O/c1-2-3
InChI key
GQPLMRYTRLFLPF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Nitrous oxide can be used as:
N2O can also be reacted with N-heterocyclic olefins (NHOs) to form electron-rich azo-bridged NHO dimers.
- A nitrogen donor to synthesize alkynyl and alkenyl substituted triazenes by coupling reaction with lithium amides and organomagnesium compounds.
- A reagent to synthesize azoimidazolium dyes.
N2O can also be reacted with N-heterocyclic olefins (NHOs) to form electron-rich azo-bridged NHO dimers.
Packaging
pressure tin
filling pressure at 15°C: 11 bar; content (15°C, 1 bar): 11 l
filling pressure at 15°C: 11 bar; content (15°C, 1 bar): 11 l
Other Notes
Sales restrictions may apply
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Ox. Gas 1 - Press. Gas Liquefied gas - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
2A - Gases
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Regulatory Information
新产品
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of organic super-electron-donors by reaction of nitrous oxide with n-heterocyclic olefins
Eymann LYM, et al.
Journal of the American Chemical Society, 141, 17112-17116 (2019)
Synthesis of triazenes with nitrous oxide
Kiefer G, et al.
Angewandte Chemie (International Edition in English), 54, 302-305 (2015)
Synthesis of azoimidazolium dyes with nitrous oxide
Tskhovrebov AG, et al.
Angewandte Chemie (International Edition in English), 127, 1305-1308 (2015)
Imke Kuiper et al.
Global change biology, 19(9), 2814-2825 (2013-04-30)
Nitrous oxide (N2 O) emissions from soils contribute significantly to global warming. Mitigation of N2 O emissions is severely hampered by a lack of understanding of its main controls. Fluxes can only partly be predicted from soil abiotic factors and
Yan Chen et al.
Anesthesiology, 118(6), 1322-1331 (2013-04-04)
Nitrous oxide inactivates methionine synthase and may lead to DNA damage and wound infection. By using single-cell gel electrophoresis (comet assay), the authors determined the effect of nitrous oxide on DNA damage in circulating leukocytes. In this double-blind, randomized controlled
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service