Protein Subunit Vaccine Manufacturing
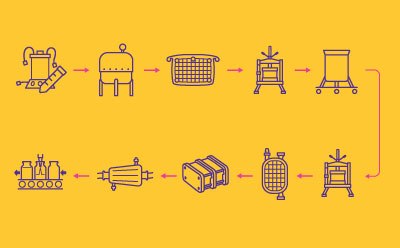
Process Train for Protein Subunit vaccines
Protein subunit vaccines use purified, recombinant fragments of viral proteins as antigens to stimulate the immune system and create protective immunity. Because viral protein fragments are incapable of causing infection, recombinant protein-based vaccines are considered to be safer than use of live attenuated or inactivated viruses. The immune response generated by protein vaccines may be weaker, however, than that produced by other vaccine types, and use of an adjuvant may therefore be necessary. Recombinant protein subunits can be produced using a variety of methods including microbial fermentation, mammalian cells, and insect cells. A robust downstream process is then required to remove any impurities and contaminants, which is followed by final sterile filtration and filling.
Featured Categories
The Mobius® Bioreactor family offers single-use bioreactors for cell culturing from early development to commercial production, supporting various scales.
Explore Mobius® single-use mixing solutions for pharmaceutical ingredient blending and process solution preparation.
Enhance filtration with our advanced tangential flow systems. Minimized hold-up, high concentration, and impeccable recovery for varied applications.
Find protein stabilizers, viscosity-reducing excipients, and granulated materials for your biopharmaceutical formulation.
Optimize upstream productivity
While different platforms are available for the upstream production of recombinant proteins for subunit vaccines, the selected approach must be optimized to achieve productivity targets, be reproducible, and scalable.
Chinese hamster ovary (CHO) cells are preferentially used for recombinant protein production as they are readily transfected and offer high protein yield. Insect cells are another option for production of recombinant proteins and are cost-effective, scalable, and less demanding than mammalian cells.
Maximize Downstream Recovery and Impurity Removal
Downstream purification to capture and concentrate the vaccine antigen and to remove process- and product-related impurities is inherently challenging and costly. Recombinant protein subunits can be particularly difficult to separate from byproducts such as truncated forms during manufacturing. As such, it is essential to have access to a range of downstream solutions to optimize the process and maximize recovery.
Ensure patient safety with sterile filtration, formulation and final fill
To help ensure patient safety, the recombinant protein subunit product must be sterile filtered using a 0.22 µm filter. Single-use components can be used for formulation of the vaccine; single-use bags containing formulation reagents can be connected to any mixer through sterile quick-connects. Following formulation, the vaccine can be aseptically transferred to single-use filling systems for final filling and vialing.
Build quality into the process
Deployment of software for data collection, monitoring, and analysis, along with integration of process analytical technology (PAT) into the manufacturing workflow, provides greater process understanding, agility, flexibility, and improved quality assurance. Raman spectroscopy is among the many tools used as PAT as it enables the molecular composition of a sample to be monitored over time which reflects critical process parameters (CPPs) and critical quality attributes (CQAs).
Visit our document search for data sheets, certificates and technical documentation.
Clarification
Clarification
Clarification is used to separate recombinant proteins from a range of impurities and prepare the feedstream for downstream processing, using pDADMAC 10% solution, new improved depth filters like Millistak+® Pod disposable depth filter system, Millistak+® HC Pro Pod Depth Filters or Clarisolve® Pod disposable depth filter
Downstream - Tangential Flow Filtration
Tangential flow filtration helps to ensure high yield, process efficiency and recombinant protein subunit recovery while contributing to impurity removal. Our portfolio include Ultrafiltration / Diafiltration with Pellicon® 2 Cassettes, ultrafiltration / Diafiltration with Pellicon® 3 Cassettes, or ultrafiltration / Diafiltration with Pellicon® Capsules, and single-use Mobius® TFF 80 systems
Downstream – Chromatography & Viral Clearance
Downstream purification to capture and/or polish chromatography with Mobius® FlexReady Solution for chromatography, and chromatography resins, polish with chromatography membranes. Concentrate the recombinant vaccine antigen, separate unwanted impurities and contaminants, and ensure viral clearance with Viresolve® Pro solutions or with Mobius® FlexReady solution for large scale virus filtration is an essential part of the process.
- Learn more on Eshmuno CMX resin, a novel mixed mode cation exchange resin for the purification of glycoproteins
Process and Formulation Chemicals
Chemicals are used all along the manufacturing process from upstream to formulation. Our portfolio include all needed buffers and salts, as well as excipients and cleaning in place chemicals. The right quality grade, together with comprehensive documentation provided by Emprove® Program are essential for qualification, risk assessment, and process optimization for vaccine development and manufacturing.
Final Sterile filtration & filling
Reliable and robust sterile filtration, formulation, and final fill of the protein subunit vaccine is essential to ensure patient safety, using Millipak® Final Fill filters, Mobius® single-use mixing solution, Mobius® 2D and 3D Assemblies and Storage Systems and Novaseptum® Go Sterile Sampling Solutions
Analytics Software & PAT Technology
Process analytical technology (PAT) and Bio4C ProcessPad™ data analytics and Bio4C Orchestrator™ automation softwares can be used to build quality into the protein subunit vaccine manufacturing processes by monitoring and controlling processes inline and in real time.
- Free eBook outlines plasmid DNA manufacturing process and downstream purification challenges for various applications.
- Adjuvant selection for vaccine formulation considers safety, effectiveness, and other characteristics for optimal application.
- Cost Modeling Vaccine Manufacturing: Estimate Production Costs for mRNA and other Vaccine ModalitiesA custom-designed cost model is used to explore the economics of vaccine manufacturing across several different modalities including mRNA. The model enables greater process understanding, simulates bottlenecks, and helps to optimize production efficiency.
- This technical article breaks down the steps of upstream and downstream bioprocessing and formulation of virus-like particle vaccines.
- See All (9)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?