L-Tyrosine in Cell Culture
What is L-Tyrosine?
L-tyrosine is one of the twenty amino acids used in protein biosynthesis. It was first discovered in 1846, when German chemist Justus von Liebig extracted it from casein, a protein found in milk and cheese. The amino acid was named after the Greek word "tyros", which means cheese. Due to its side chain, L-tyrosine also belongs to aromatic, polar and hydrophobic amino acid group.
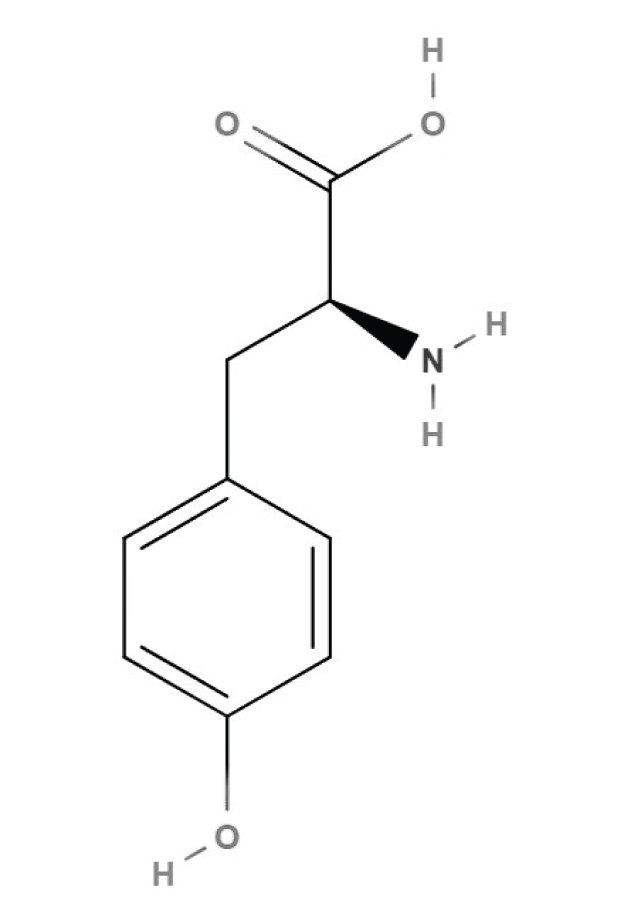
Figure 1.Structure of L-tyrosine
L-tyrosine is considered a non-essential amino acid as it can be produced by the body. In mammals, L-tyrosine is converted from L-phenylalanine, an essential amino acid. Phenylalanine is derived from food by the enzyme phenylalanine hydroxylase (EC 1.14.16.1).
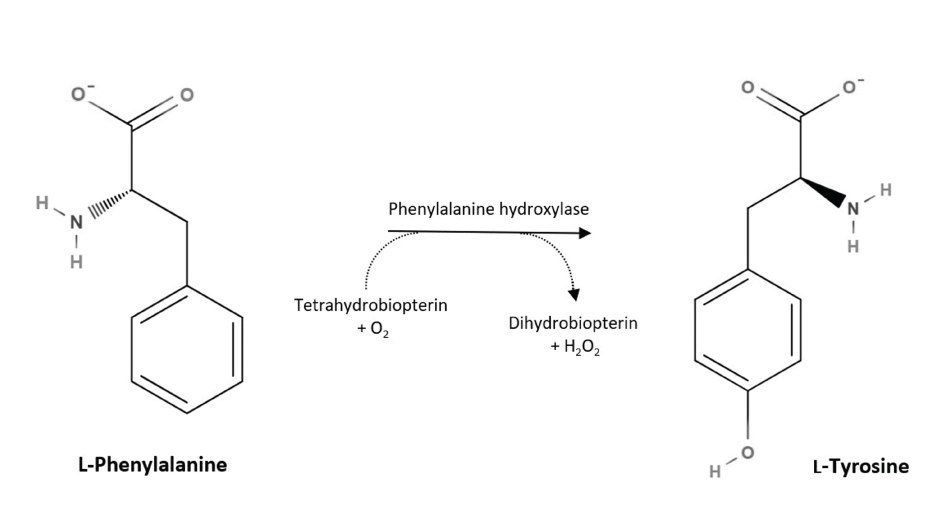
Figure 2.Biosynthesis of L-tyrosine in mammals
L-tyrosine is widely recognized as an important amino acid for cell growth and viability. L-tyrosine is incorporated into proteins and other biologically important molecules such as neurotransmitters, hormones, pigment melanin and coenzyme Q10.
Related Products
Function of L-Tyrosine
L-tyrosine helps shape the 3D structure of protein through its interactions with other amino acid residues within the protein. The phenolic ring of tyrosine is partly hydrophobic and polar, giving L-tyrosine both hydrophobic and hydrophilic qualities.
These physicochemical properties make L-tyrosine the most effective residue for mediating molecular recognition. Tyrosine residues can be positioned at protein surfaces, binding interfaces, or buried within a protein. It is often enriched on the protein surfaces that bind small molecules, nucleic acids, or protein partners, and plays critical roles in a protein’s interaction with its partners1,2.
Modifying L-tyrosine can dramatically alter the structure of a protein. Tyrosine can be phosphorylated, converting it to phosphotyrosine. This phosphorylation is the key step in cell signaling transduction, cell migration differentiation, the cell cycle, gene regulation, and nerve transmission.
Tyrosine can also be sulfated, in a process known as tyrosine sulfation. This modification is essential for several biological processes, including proteolytic activation of coagulation factors V and VIII by thrombin and proteolysis of the complement C4a-chain by C1s. Tyrosine sulfation of the HIV-1 co-receptor CCR5 is also required for viral entry into host cells.
Both the phosphorylation and sulfation modifications can be detected via antibody.
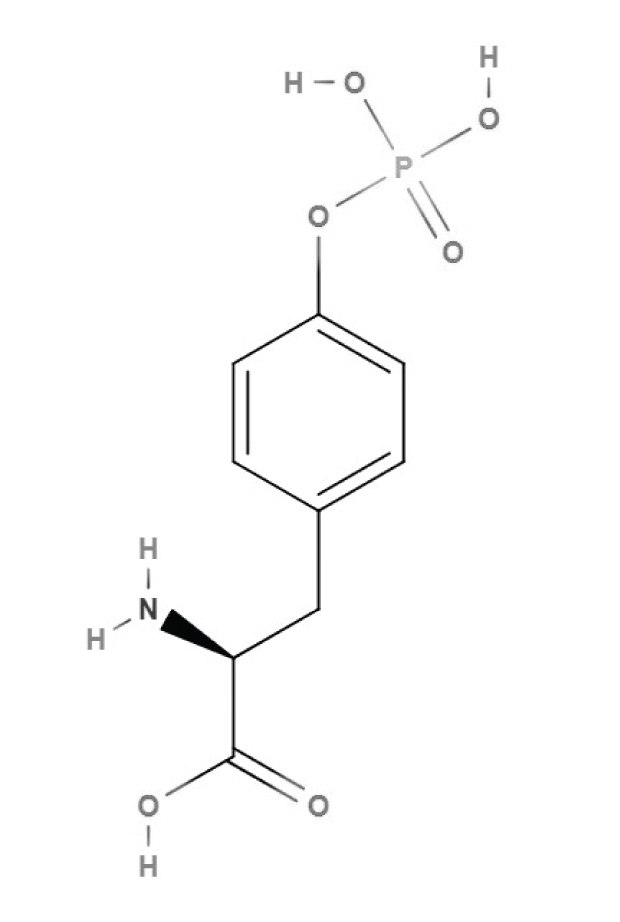
Figure 3.Structure of phosphotyrosine
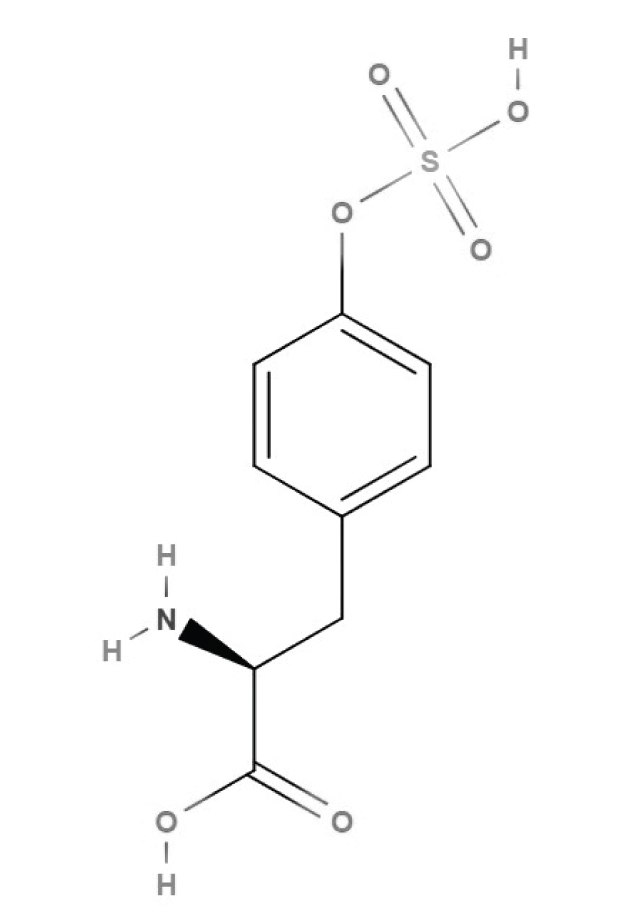
Figure 4.Structure of sulfotyrosine
L-tyrosine plays several critical roles in the body besides its role as a proteinogenic amino acid. It acts as a precursor for the biosynthesis of several neurotransmitters including dopamine, norepinephrine, and epinephrine3. It is also the initial substrate of melanogenesis4 and coenzyme Q10 biosynthesis5. In the thyroid gland, L-tyrosine from thyroglobulin synthesizes thyroxine, an important hormone for regulating metabolism by sequential iodination of the tyrosine phenol rings6. Tyrosine residues in membrane protein can also help protect the membrane integrity of cells from oxidative stress7.
L-Tyrosine in Cell Culture
L-tyrosine, as a non-essential amino acid, can be produced by cells. However, non-essential amino acids can still be added to the cell culture medium to increase the growth and viability of the cultures. When non-essential amino acids are added to the media, the cells can preserve more energy, gain nutrition, and avoid the toxic by-products produced during biosynthesis. L-tyrosine is one of the amino acid components in many cell culture media including MEM, DMEM, and RPMI. For some cell lines, additional supplementation of L-tyrosine is still necessary.
L-tyrosine supplementation is important in monoclonal antibody (mAb) production using Chinese hamster ovary (CHO) cell cultures8. Insufficient L-tyrosine supply reduces the specific productivity and can cause tyrosine sequence variants - tyrosine residues replaced either by phenylalanine or histidine. It is necessary to optimize the concentration of L-tyrosine, along with other amino acids, for all the large-scale monoclonal antibody productions.
Reactive oxygen species (ROS) are the natural byproducts of aerobic metabolism. ROS are highly reactivity towards protein, lipids, RNA, and DNA and impact cellular health when their levels are too high9. L-tyrosine can amplify the riboflavin‐dependent ROS production10. L-tyrosine supplementation should be well optimized when culturing the cells sensitive to oxidative stress, such as stem cells and some cancer cell lines.
Tyrosine-supplemented agar plates are also used to culture bacteria, including some species of Bacillus, Nocardia and Streptomyces.
L-Tyrosine Properties in Cell Culture Media
L-tyrosine has a very low solubility (0.45mg/ml) in water at neural pH. To prepare a stock solution, L-tyrosine is dissolved at an extreme pH, either below pH 2 or above pH 9. It can also be dissolved in organic solvent such as DMSO with higher solubility11.
L-tyrosine disodium salt dihydrate has a much better solubility (100mg/ml) when compared to L-tyrosine. To avoid introducing pH shock, un-wanted solvent, or high salt into the medium, dipeptides that contain L-tyrosine, such as glycyl-L-tyrosine, are a reliable replacement when high concentrations of L-tyrosine are required.
Chemical Characteristics Of L-Tyrosine
L-Tyrosine has the molecular formula C9H11NO3 and molecular weight of 181.191 g/mol. It has an isoelectric point of 5.63 and pKa of 2.2 and 9.21.
Tyrosine is a relatively stable amino acid. However, tyrosine will be subjected to halogenation if there is a high concentration of HCl is present in solution. It is important to use fresh and properly stored tyrosine to ensure maximum potency and effectiveness when using L-tyrosine in cell culture or other biological applications.
References
To continue reading please sign in or create an account.
Don't Have An Account?