A Comparison of Solid Phase Extraction (SPE) Products Used to Purify Peanut Paste Extract for Aflatoxin Analysis
Introduction
Aflatoxins are secondary metabolites produced by fungi of the Aspergillus genus. Two Aspergillus species, A. flavus and A. parasiticus have been widely studied because they significantly impact commercial agriculture.1 These species commonly infect cereal grains, especially maize, as well as ground and tree nuts. Aflatoxins are toxic to livestock and can retard growth and interfere with reproduction. In humans, aflatoxin exposure may reduce immune response. More critically, aflatoxin AFB1 has been classified by the International Agency for Research on Cancer (IARC) as a group 1 (known) human carcinogen.2,3,4 On the basis of these and other findings, the presence, and concentration of aflatoxins in agricultural commodities is widely regulated. European Union treaty countries, for example, have stringent aflatoxin testing criteria. The EU’s Commission Regulation (EC) No 1881/2006 sets a maximum of 0.1 μg/kg concentration for AFB1 used to produce cereal products for infants and children.5 Solid Phase Extraction (SPE) is a popular choice for sample cleanup prior to HPLC or LC/MS analysis of aflatoxin samples. Broadly, two modes of SPE are employed: bind and elute and interference removal. Generally, bind and elute SPE modes require multiple steps including conditioning, equilibration, washing, and elution while interference removal SPE modes may only require passing a sample aliquot through an SPE cartridge using a vacuum.6,7 For routine sample cleanup and high throughput, interference removal SPE is the preferred choice. The following study compares the cleanup performance of five SPE products using the interference removal cleanup.
Experimental
Sample Extraction
Aflatoxin-free peanut paste (50 g) was added to 200 mL of 84:16 acetonitrile: water and extracted for 3 minutes using a variable-speed blender at approximately 15,000 rpms. The blended mixture was filtered through qualitative filter paper using a vacuum flask and ceramic funnel. Filtered extract was transferred to a glass container and allowed to stand overnight allowing for the separation of peanut oils from the aqueous extract. After standing, the aqueous sample was decanted into 50 mL conical centrifuge tubes and centrifuged at 5000 rpm (RCF = 4863) for 5 minutes. The resulting supernatant was used for matrix sample blanks and spikes.
SPE Cleanup
Supel™ Tox AflaZea SPE was compared to four competitor SPE products designed for aflatoxin cleanup. The four competitor products are referred to as "C1," "C2," "C3," and "C4." All compared products achieve sample cleanup by interference removal. Samples were processed according to manufacturer specifications and were collected and derivatized for analysis on the day processed. Loading aliquots were pulled through SPE cartridges at 2-3 drops/second by means of a vacuum manifold. SPE cartridges and loading details are identified in Table 1.
Sample Derivatization
For each analyzed sample, 200 μL of cleaned matrix spike was pipetted into a 5 mL microreactor vessel. Derivatization solution (300 μL), consisting of 70:20:10 water: trifluoroacetic acid: acetic acid, was then added to the matrix sample and the mixture was heated at 65 °C for 25 minutes using a hot plate and heating block. The derivatized sample was then removed from the block and cooled to ambient temperature. A volume of 500 μL of mobile phase (20:80 acetonitrile: water) was then added to the mixture bringing the final sample volume to 1 mL. Finished samples were vortexed 30 seconds and transferred to polypropylene autosampler vials for HPLC analysis with fluorescence detection.
Sample Spiking
A 100 mL volumetric flask was partially filled with sample extract then spiked with 100 μL of Aflatoxin Mix 4 solution (Product No. 34036). The flask was then filled to the line and stirred rapidly for approximately 20 minutes using a magnetic stir bar and plate. For reference purposes, 84:16 acetonitrile: water was spiked as matrix samples with Aflatoxin Mix 4 solution; using the sample spiking procedure as used for matrix samples and derivatized for analysis. Reference samples were not passed through an SPE column prior to analysis.
Quantification
Calibration curves were used to calculate final aflatoxin concentrations. Curves were constructed using the Aflatoxin Mix 4 solution used for matrix spikes. The mixture contains aflatoxins at the following concentrations: 2 μg /mL of B1 and G1, and 0.5 μg/mL of B2 and G2. A 5 mL aliquot of 84:16 acetonitrile: water was spiked with 15 μL of the Aflatoxin Mix 4 solution resulting in final concentrations of 24 ng/mL for aflatoxins B1 and G1 and 6 ng/ mL for aflatoxins B2 and G2. Calibration curves for aflatoxins B1 and G1 were constructed using 6, 12, 15, 21, and 24 ng/mL concentrations, while those for B2 and G2 were constructed using 1.5, 3, 3.75, 5.25, and 6 ng/mL concentrations.
Results and Discussion
All SPE products compared were sourced from major suppliers. Peanut matrix was considered particularly relevant because peanuts are an important protein source in developing nations where climate conditions favorable to Aspergillus infection and aflatoxin production are common.8 Aflatoxins B1 and G1 are necessarily derivatized to increase fluorescence. Several methods can be used to accomplish the derivatization. A trifluoroacetic acid (TFA) method was used for its simplicity, reliability, and because the process requires no specialized apparatus. The HPLC method used was optimized for sensitivity by selecting an Ascentis® Express column and by refining FLD excitation and emission wavelengths for a response. Quantitation of aflatoxin concentrations was achieved by the use of calibration curves. The linearity for all calibration curves was greater than r2= 0.99. Chromatograms of all spiked samples after processing with the respective SPE cartridges are shown in Figure 2.
At the aflatoxin concentration levels tested, EU standards require analyte recoveries ranging from 70% to 120%.3 Recoveries for G2 using products C3 and C4 were outside the range (Table 2, Figure 1). All other products used met the specified recovery criteria. Supel Tox AflaZea analyte recovery values ranged from 92% to 107% with RSD’s from 1% to 7% (Table 2).
The format (large bed size) of products C1 and C2 required pipetting two 3 mL sample aliquots into each SPE cartridge. The Supel Tox AflaZea SPE cartridges, as well as products C3 and C4, required a single sample aliquot. Supel Tox AflaZea required 2 mL of sample for cleanup, a significantly lower volume than used in any competitor cartridge (Table 1).
HPLC analysis of all spiked samples after SPE cleanup showed that all compared SPE cartridges effectively eliminate interferences. Analysis of products C3 and C4 resulted in unacceptably high analyte recoveries of aflatoxin G2. Supel Tox AflaZea SPE produced exceptional analyte recoveries with good reproducibility for all aflatoxins while requiring less than half the sample volume required by competing products.
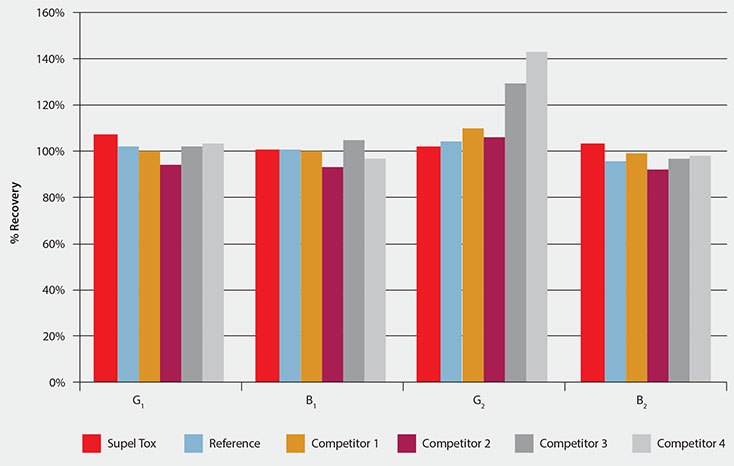
Figure 1.Bar Chart Illustrating Average %Recovery for Peanut Paste Spiked with Aflatoxin G1 (8 ppb), B1 (8 ppb), G2 (2 ppb), and B2 (2 ppb), n=3

Figure 2.HPLC/FLD Analysis of Aflatoxins in Raw Peanut Paste, Spiked with Aflatoxin G1 (8 ppb), B1 (8 ppb), G2 (2 ppb), and B2 (2 ppb). (a) Reference in acetonitrile:water (84:16) (b) Supel Tox AflaZea Sample Spike; (c) Competitor 1 Sample Spike; (d) Competitor 2 Sample Spike; (e) Competitor 3 Sample Spike, and (f) Competitor 4 Sample Spike.
Conclusion
In terms of analyte recovery, reproducibility, sample utilization and ease-of-use, the Supel Tox AflaZea SPE product outperformed the comparison products.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?