Analysis of Drugs of Abuse in Urine

Introduction
Supel™ Swift HLB SPE is a new, proprietary, and patent pending copolymer having both hydrophilic and lipophilic functional groups. It is intended for use as a sorbent material in solid phase extractions (SPE) prior to instrumental analysis, such as LC-MS/ MS. The dual polarity of Supel™ Swift HLB makes it ideal for extracting a broad range of compounds from aqueous matrices and is appropriate for samples in food & environmental applications as well as biological samples such as urine, serum, and plasma. The hydrophilic and lipophilic balanced (HLB) property of the polymer material enables the retention of a broad spectrum of compounds having a wide range of polarities and log P values (-0.9 - to 4.7).
In this study, we demonstrate the cleanup of urine samples by HLB solid phase extraction for the analysis of opioids via LC-MS/ MS. The 96-well SPE format (Figure 1) utilized is optimal for clinical and other laboratories working in a high-throughput environment.

Figure 1.The Supel™ Swift HLB 96-well plate, 30 mg of HLB sorbent/well.
During the analysis of drugs of abuse in urine, the drug and their metabolites (e.g. morphine) can be present glucuronide form (Figure 2). In these cases, hydrolysis using a β-glucuronidase enzyme is performed prior to LC-MS analysis of the samples to ensure that the free form of the drug can be analyzed in the samples under investigation. Subsequently, the samples require a cleanup prior to injection into the LC-MS instrument. Solid phase extraction remains the most convenient method for use in such sample cleanups.

Figure 2.β-Glucuronidase hydrolysis of morphine-3-β-D-glucuronide to morphine.
Methods
Recovery of Analytes
Synthetic urine, SigMatrix Urine Diluent, was spiked with “Pain Management Multi-Component Opiate Mixture-13 solution” diluted to 100 ng/mL for 12 of the 13 compounds and at 10 ng/mL for fentanyl. A list of the components and the transitions monitored is available in Table 1. The following internal standards were added at 10 ng/mL: oxycodone-D3, (±)-methadone-D9, oxymorphone-D3, hydrocodone-D3, cis-tramadol-13C, D3, meperidine-D4. The MS transitions monitored with these internal standards are shown in Table 2.
A β-glucuronidase solution at a concentration of 10 kU/g was prepared in 0.1 M phosphate buffer (pH 6). The bulk sample solution comprised of 3:1:1 SigMatrix urine diluent:β-glucuronidase (10 kU, pH 6.0):phosphate buffer (pH 6.0). The samples underwent digestion for 2 hours at 60 oC with mixing at 200 rpm. The hydrolysis conditions used were previously found to be optimum for using β-glucuronidase enzyme from limpets. The samples were cooled, and the sample solutions adjusted to pH 9 with an ammonium hydroxide solution. The samples were then processed on a Supel™ Swift HLB 96-well plate containing 30 mg/well of HLB sorbent as outlined in Figure 3. After sample processing, 75 µL of cleaned sample was diluted with 175 µL of LC/MS grade water to decrease the final organic component to 30%. Samples were analyzed on a Sciex 3200 QTrap MS instrument with an Agilent 1290 LC (separation parameters are shown in Table 3). Analytes were quantified by a 5-point external calibration curve using standards prepared daily from methanol stock solutions stored in glass vials. Injected calibrator solutions contained 10 ng/mL of the previously outlined internal standards in 70:30 methanol:water.
Matrix Effects on Ionization
Samples were prepared and processed as described earlier except for the spiking of analytes and internal standards. The cleaned matrix was spiked after processing with both analytes and internal standards. These samples were quantified by a 5-point external calibration curve as described earlier.
Results and Discussion
Percent Recovery
A representative chromatogram of an SPE cleaned- up sample spiked at 100 ng/mL (except for fentanyl at 10 ng/mL) is shown in Figure 4. Overall, 12 of the 13 analytes showed relative recoveries of 73 to 105% (n=96) with an overall recovery of 88% as shown in Table 4 and Figure 5. The lower recovery for buprenorphine is attributed to a log P ~5, which would have led to non-specific binding.
For the thirteen analytes, the RSDs associated with the recoveries were <7.2% (n=96) showing consistency across the plate. Absolute recoveries are shown in Figure 6.
Without using the assigned internal standard, the absolute recovery across the plate for 12 of the 13 analytes was 70.5% (omitting buprenorphine). Nine of the 13 analytes show recovery at ≥70% as shown in Figure 6 across the 96 wells.
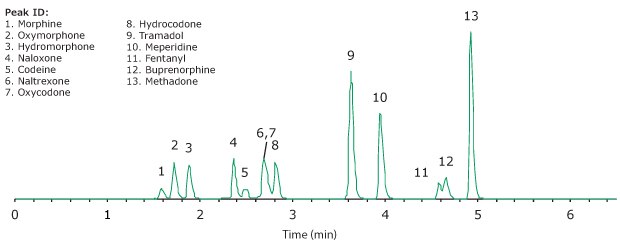
Figure 4.Representative chromatogram of the spiked urine-mimic samples after cleanup with SPE.
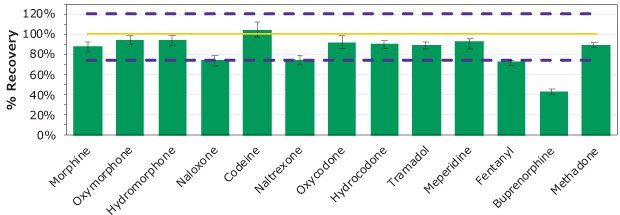
Figure 5.Relative percent recovery. Analytes were spiked at 100 ng/mL with exception of fentanyl at 10 ng/mL. Purple dash lines represent 75 and 120% recovery, with the yellow solid line representing 100% recovery. Analytes are listed in elution order.
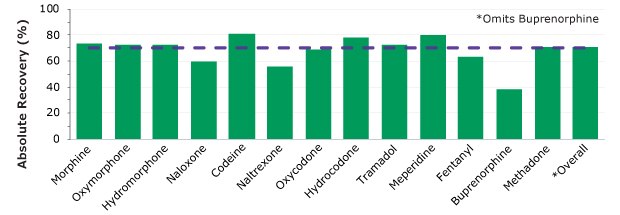
Figure 6.Absolute percent recovery Analytes were spiked at 100 ng/mL with exception of fentanyl at 10 ng/mL. Purple dash lines represent 70% absolute recovery. Analytes are listed in elution order.
Matrix Effects
The impact of matrix components was calculated by comparing the signal response of the analyte in 70:30 methanol:water (representing 100%) to a sample that was cleaned using the SPE procedure outlined and was post-spiked (final extracted samples had 30% methanol present). Across the 13 analytes, minimal to no matrix effects (suppression or enhancement) ±10% was observed for most of the analytes as shown in Figure 7. Two analytes that were suppressed the most were naloxone (-30%) and naltrexone (-20%). These suppression values would have lead to the lower absolute recovery reported in Figure 6 but were corrected for in relative recovery by use of an internal standard (Figure 5).
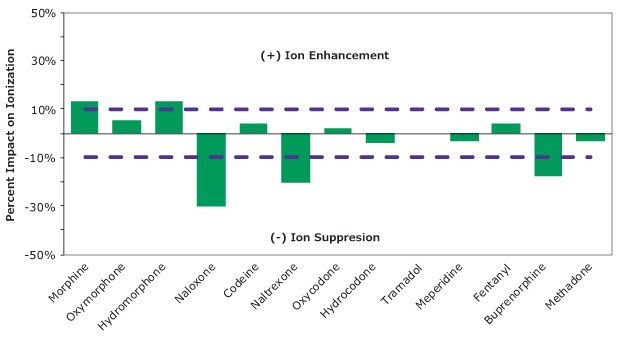
Figure 7.Matrix effects (ion suppression and ion enhancement) across the 13 analytes. Purple dash lines represent ±10% impact on ionization.
Summary
Supel™ Swift HLB SPE is a hydrophilic and lipophilic polymer SPE phase designed for the extraction of a broad range of compounds from complex aqueous sample matrices. In this study, we demonstrated the utility of this SPE phase to prepare urine samples for the analysis of a series of pain management drugs readily available as a premade mixture. No post- extraction concentration or dry down of samples was required. The relative recoveries of the analytes were in the range of 73-105% with one exception of buprenorphine. The reproducibility across the entire plate was excellent with ≤7.2% RSD. Minimum matrix effects (±10%) were observed after Supel™ Swift HLB SPE cleanup. The developed SPE method can be applied to analyze a wider range of analytes in urine.
More information on clinical testing is available at SigmaAldrich.com/clinical
SPE & HPLC
Solvents & Reagents
Accessories
Certified Reference Materials (Cerilliant®)
To continue reading please sign in or create an account.
Don't Have An Account?