Pesticide Residue Analysis in Ginger Powder with QuEChERS
Introduction
Ginger comes from the rhizome of the plant Zingiber officinale. It has been used for thousands of years as both a spice for cooking and a treatment for ailments such as nausea, gastrointestinal (GI) irritation, inflammations and cold & flu symptoms. In addition, recent studies indicate that it may be useful in pain reduction and supporting cardiovascular health.1 Ginger is native to southeast Asia, however it is also grown in various countries in the western hemisphere. The world production of ginger was approximately 2.1 million metric tons in 2013, with about half coming from China and India.2 After harvesting, ginger root is first washed and then boiled in a process known as “killing”, which stops enzymatic activity. It is then dried and subjected to further processing such as grinding for powdered ginger, or solvent extraction and distillation for production of ginger oil and oleoresin.3 Many pesticides used on the plant can be carried through these processes and end up in the final product. Since dried ginger is used for both cooking and as a dietary supplement, there is a risk for exposure to pesticides as a result of more frequent consumption. Thus, pesticide residue analysis of ginger is required by many countries. For example, Canada has maximum residue limits for a variety of pesticides in ginger root ranging from 0.01 to 0.15 ppm.4
The odor and pungency of ginger is due to the presence of terpenes, gingerols and shogaols.5 These compounds contribute to the highly complex matrix of ginger, which subsequently presents a special challenge in low level analyses of contaminants. The analysis of pesticide residues in ginger can be achieved using the “Quick, Easy, Cheap, Effective, Rugged and Safe” (QuEChERS) approach for extraction. Following extraction, a cleanup step is essential to produce a sample that is amenable to both LC and GC chromatographic analysis. A common approach, included in the QuEChERS methodology is to use dispersive solid phase extraction (dSPE) with loose sorbents. However, dry commodities such as ginger powder often produce too much background for effective cleanup using dSPE. A traditional solid phase extraction (SPE) cartridge has more cleanup capacity than dSPE, and is recommended for these types of samples. In this application, a new dual-layer SPE cartridge, the Supelclean™ Ultra 2400, was used in the cleanup of QuEChERS extracts of ginger powder prior to analysis of pesticide residues by both LC-MS/MS and GC-MS/MS. This cartridge contains a blend of primary-secondary amine (PSA), C18, and graphitized carbon in the top layer and Z-Sep sorbent in the bottom layer. The cartridge contains sorbents traditionally used for cleanup (PSA and C18) as well as two novel materials; Graphsphere™ 2031 and Z-Sep. Graphsphere™ 2031 is a specially engineered carbon with a lower surface area than standard graphitized carbon black (GCB). The lower surface area provides a balance between removal of pigments with planar structures such as chlorophyll, and weaker retention of planar pesticides. The Z-Sep in the bottom layer is zirconia coated silica which can retain carotenoids as well as some fatty constituents. Together these two layers of sorbents provide more rigorous cleanup than dSPE. In addition, compared to current dual layer SPE cartridges containing GCB and PSA or aminopropyl silica, the Ultra 2400 is much smaller, but still provides sufficient sample cleanup with much less solvent consumption. The use of Graphsphere™ 2031 carbon in the Ultra 2400 offers an advantage over traditional GCB containing cartridges in the form of improved recoveries of planar pesticides without the use of toluene in the elution solvent.
In this study, ginger powder was spiked at 10 ng/g with a variety of pesticides, and extracted using a standard QuEChERS approach. The extract was then cleaned for LC-MS/MS and GC-MS/MS analyses using the 3 mL Supelclean™ Ultra 2400 SPE cartridge.
Experimental
Dry ginger powder was obtained from a local grocery store, and spiked at 10 ng/g with the pesticides listed in Tables 1 and 2. Samples were extracted as described in Figure 1 and subjected to separate cleanups for LC and GC as described in Figure 2. Spiked replicates of ginger extract (n=3) and blanks were subjected to cleanup using the described procedures. Samples were analyzed by external standard analysis against matrix- matched calibration curves using the HPLC and GC conditions shown in Tables 3 and 4. The MRMs used for quantitation are indicated in Tables 1 and 2.
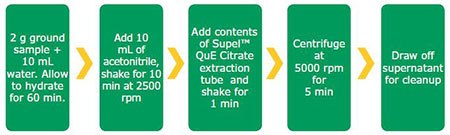
Figure 1.QuEChERS extraction procedure used for ginger powder

Figure 2.Cleanups used for QuEChERS extracts of ginger powder using 3 mL Supelclean™ Ultra 2400 cartridge.
*ammonium adduct
Results & discussion
Background
After cleanup, the extract prepared for GC analysis showed substantially less color (Figure 3). For comparison, an aliquot of extract was also cleaned by dSPE using PSA/C18/GCB and PSA/C18. The PSA/ C18/GCB and Ultra 2400 cleaned extracts were similar in color, while the extract cleaned using PSA/C18 was darker yellow, indicating the need for carbon to reduce pigmentation. GC/MS scan comparisons of the Ultra 2400, PSA/C18/GCB cleaned and uncleaned extracts are shown in Figure 4. Peak patterns are similar between the three extracts, however the overall amplitude of the peaks was reduced after cleanup. Most of the background peaks eluting prior to 15 minutes were removed by the Ultra 2400 cleanup. The reduction in background, measured as total peak areas, was 11% using PSA/C18/GCB and 21% using Ultra 2400.

Figure 3.QuEChERS extracts of ginger powder after cleanup by dSPE and Supelclean™ Ultra 2400 SPE
Pesticide recovery
The pesticide recoveries and reproducibilities obtained after cleanup with Ultra 2400 are presented in Table 5 and Figure 5. The average recovery obtained was 82%, with an average RSD of 9%. Of the 51 pesticides, 38 were within the 70-120% recovery range considered acceptable. Recovery of permethrin and uniconazole-P were affected by matrix interference. Procymidon was a very poor responding analyte by LC-MS/MS, and was difficult to detect at 10 ng/g. It is suspected that recoveries of boscalid, carbendazim, diflubenzuron, nitralin and tetraconazole were reduced by the amount of sorbent to which the extract was exposed during cleanup. In past work done by the authors with cleanup of turmeric extract using the 3 mL Ultra 2400 SPE cartridge, these same pesticides had recoveries of >70%. Turmeric is much more oily and pigmented than ginger, and thus there was more matrix available to bind with active sites on the sorbents in the SPE cartridge. In the case of ginger, the relatively lower amounts of oil and pigment could have resulted in increased binding of these pesticides with the sorbents. A smaller 1 mL Ultra cartridge containing less sorbent weight could be used to increase recoveries.
Dichlorvos exhibited very low and variable recovery, and this same behavior has been observed by others using zirconia-based sorbents for cleanup.6 This is most likely due to Lewis acid/base interaction between the Z-Sep and the phosphate group present in the structure of dichlorvos. Other pesticides containing phosphate groups: fosthiazate, methamidophos, mevinphos and monocrotophos, had better recoveries. All were recovered at >70%, except mevinphos, which was recovered at 57%. Both mevinphos and dichlorvos were analyzed by GC-MS/MS while the other phosphate containing pesticides were analyzed by LC-MS/MS. The difference in recoveries could be due to the variation in elution protocols used during the cleanups. Elution from the Ultra 2400 cartridge for GC analysis used acetonitrile containing formic acid, while LC used methanol:acetonitrile containing ammonium formate. Formic acid is a Lewis base, and is added to prevent interaction between the Z-Sep and weaker Lewis bases such as some acidic compounds. However, this was not entirely effective in the case of the phosphate groups in dichlorvos and mevinphos. The ammonium formate in the LC elution solvent acts as a Lewis base, and also acts to disrupt any weak cation exchange interactions that may be occurring between basic compounds and silanol groups present in the silica of the Z-Sep.7 The addition of methanol to the elution solvent may also be contributing to increased recovery of the phosphate containing pesticides. It has been shown that methanol addition increases pesticide recovery when using Z-Sep sorbent; possibly by disrupting Lewis acid-base and/or electrostatic interactions.8
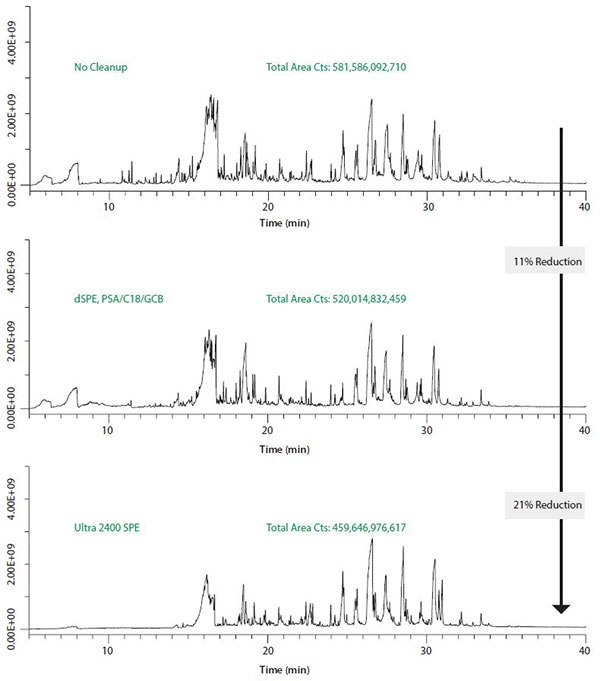
Figure 4.GC-MS scan analyses of ginger powder extracts.
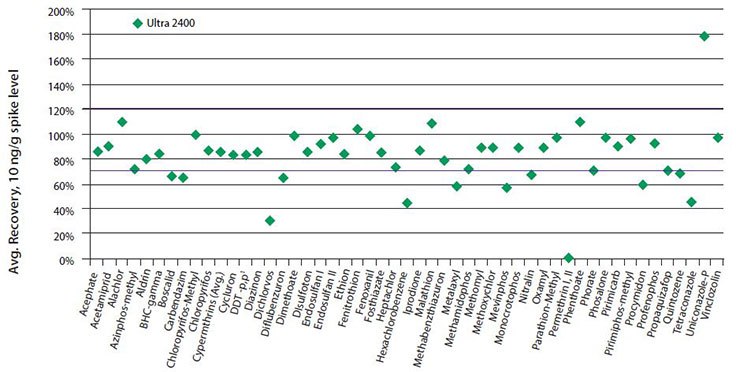
Figure 5.Average recoveries of pesticides from ginger powder spiked at 10 ng/g after cleanup with 3 mL Supelclean™ Ultra 2400 SPE.
Recovery of the planar pesticide hexachlorobenzene (HCB) is traditionally problematic after cleanup using GCB. This compound is retained by conventional GCBs during cleanup. If using SPE cleanup, toluene in the elution solvent can increase recovery of this compound by displacing it from the carbon. However, toluene is then in the final extract, making it incompatible with HPLC analysis. Recovery of HCB after cleanup with the 3 mL Ultra 2400 cartridge was 45%. This is in the same range as recoveries reported by others for cleanup of acetonitrile extracts of botanicals using 6 mL dual layer SPE cartridges in combination with toluene-containing elution solvent.9,10 To determine if recovery of HCB could be improved, the ginger extract was also cleaned using a smaller 1 mL Ultra 2400 cartridge. The elution protocol used was similar to that used for the 3 mL, with smaller solvent volumes. Recovery increased to 63%, indicating that, similar to other pesticides with recoveries <70%, the smaller 1 mL cartridge may provide a better balance between matrix and sorbent active sites for the cleanup of powdered ginger extract.
Conclusions
QuEChERS extraction of powdered ginger produces an extract containing enough background to require cleanup prior to chromatographic analysis. The use of Ultra 2400 SPE reduced this background, as evidenced by visual appearance of the extract and GC-MS-scan data. The greater capacity of the SPE cleanup reduced background more than dSPE using PSA/C18/GCB. Recoveries for 51 target pesticides, spiked at 10 ng/g, were in the range of 70-120% for 75% of the analytes. Recoveries of some of pesticides outside of this range could be improved through adjustments to the cleanup method such as use of a smaller, 1 mL Ultra SPE cartridge, and/or modifications to the elution solvent used. Compared to cleanup using larger 6 mL dual layer SPE cartridges containing GCB, the method using the 3 mL Ultra 2400 requires significantly less solvent: 11 mL vs. 20-30 mL. Recovery of the planar pesticide hexachlorobenzene was achieved without toluene in the elution solvent. Using the 3 mL cartridge, recovery from ginger was 45%; and this could be increased with use of the smaller 1 mL cartridge.
In summary, Supelclean™ Ultra SPE offers a cost effective alternative to the use of conventional 6 mL dual layer SPE containing GCB. The cartridge is available in 1 and 3 mL sizes, offering a choice to accommodate differing matrices and analyte lists.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?