ENVI-Carb Plus for the Extraction of Propylene Glycol and Ethylene Glycol from Water
Read more about
Introduction
Propylene glycol has a wide variety of applications, including uses as a solvent, emulsifier, and moisturizer in various chemical, food, and personal care products. Its toxicity to both humans and animals is relatively low. Ethylene glycol, which is widely used as automotive antifreeze, is moderately toxic and was included on the Drinking Water Contaminant Candidate List 3 Draft by EPA in 20081. Due to the hydrophilic nature of ethylene and propylene glycol, traditional methods of extraction such as liquid/liquid and solid phase extraction (SPE) with most typical sorbents, cannot extract these compounds from aqueous samples. As a result, the standard method of analysis in water samples has been direct aqueous injection into a gas chromatograph (GC). This method, however, has numerous problems, including high detection levels, carryover, and chromatographic issues. For this reason, it would be advantageous to extract these glycols into an organic solvent for GC analysis. However, as stated previously, extracting them from an aqueous matrix is difficult. Since ethylene and propylene glycol are extremely polar, reversed phase and normal phase sorbents such as C18 and silica gel cannot retain them from water. Carbon sorbents, depending on their surface and structure, can retain analytes based on hydrophobicity and molecular size and shape. These unique characteristics give carbons the potential for use as sorbents to extract small glycols from water.
ENVI-Carb™ Plus is a microporous amorphous carbon molecular sieve. Its surface is less hydrophobic than other types of carbons, which gives it a higher affinity for water, and helps to draw analytes from aqueous solution into its pore structure. Elution of analytes is achieved by flooding the pores with a solvent in which the analyte is soluble. The efficiency of extraction is increased by the material’s inert surface and minimal interstitial space (space between the individual particles). ENVI-Carb Plus was designed with a narrow particle size distribution that reduces interstitial space, thus allowing elution solvents to more thoroughly solvate the carbon’s pore structure.
ENVI-Carb Plus was developed for the extraction of highly polar compounds from water, and has been found to work well for acephate, phenol, acrylamide, and 1,4-dioxane. In the case of 1,4-dioxane, it has been validated for use in US EPA Method 522, which details the extraction and analysis of this compound in drinking water 2. Considering the retention characteristics of ENVI-Carb Plus, it was considered to have potential for extracting glycols from water. In this study, we evaluated the use of this carbon for the extraction of ethylene and propylene glycol from water. The goal was to determine if the glycols could be retained from water and eluted with an organic solvent, thus allowing for easier GC analysis and subsequent sample concentration and solvent exchange if desired. Using the protocol described, ENVI-Carb Plus was found to retain both glycols from water, and exhibited good recovery of propylene glycol from water and fair recovery of ethylene glycol.
Experimental
Samples of deionized water were spiked at varying levels with propylene and ethylene glycols. Extraction was done with ENVICarb Plus reversible (these cartridges are fitted with female Luer inlets) cartridges using the protocol described in Table 1. Prior to the elution step, the cartridge was reversed. Just enough elution solvent was then drawn through the cartridge to wet the packing, and the vacuum was turned off and the cartridge was allowed to soak for 1 minute. The vacuum was then turned back on, and the remaining elution solvent was drawn through the cartridge and collected. GC analysis (Table 2) was performed directly on the extracts without further concentration or solvent exchange.
Results and Discussion
As stated previously, aqueous injections are problematic in GC. Figure 1 shows the result of an injection of a water sample containing 25 μg/mL of propylene and ethylene glycol. Water forms an extremely large vapor cloud in a heated GC inlet, and has a high boiling point compared to other solvents. As a result, sample focusing becomes difficult, which in turn affects peak shape and response. By comparison, if the glycols are injected in the organic solvent mixture used for elution of the ENVI-Carb Plus cartridges, as shown in Figure 2, peak shape and response are improved. For this reason, it was determined that GC analysis would be suitable directly after the elution step, and that no solvent exchange step was necessary.
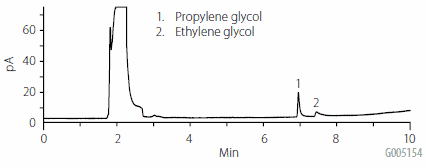
Figure 1.Direct Aqueous Injection of 25 μg/mL Spiked Water Sample
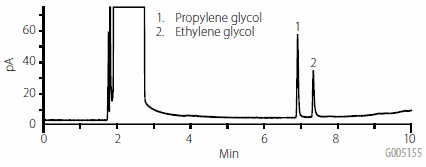
Figure 2.Injection of 25 μg/mL Glycol Standard in 50:50 Methanol:Methylene Chloride
Propylene glycol retained well on ENVI-Carb Plus, while ethylene glycol, being smaller and more hydrophilic, did not retain as well. Replicate water samples spiked at 25 μg/mL were extracted and compared to the same concentration standard in elution solvent. As shown in Table 3, reproducibility was very good overall. Recovery of propylene glycol was significantly better than the more hydrophilic ethylene glycol. A chromatogram of an extracted spiked water sample is presented in Figure 3. The response and peak shape of propylene glycol were significantly improved over direct aqueous injection. The ethylene glycol, due to low recovery and response, did not show as dramatic an improvement.
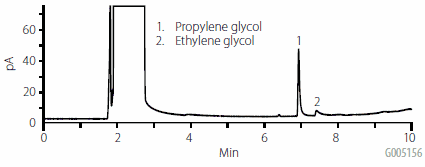
Figure 3.Injection of 25 μg/mL Water Sample Extracted Using ENVI-Carb Plus
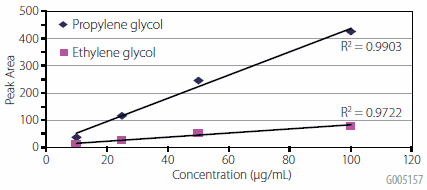
The quantitative performance of the method was evaluated by determining the linearity of extracted water samples spiked at 10 μg/mL, 25 μg/mL, 50 μg/mL, and 100 μg/mL. A plot of concentration vs. response is presented in Figure 4. Linearity was good for both glycols, and % RSD for the average response factors were 11% and 16% for propylene and ethylene glycol respectively.
Conclusions
ENVI-Carb Plus reversible cartridges were able to extract both propylene and ethylene glycol from water, and the method demonstrated to be both quantitative and reproducible. Elution was achieved with a combination of organic solvents, offering further options for GC analysis such as sample concentration or solvent exchange. Direct injection of the elution solvent offered an improvement in response and peak shape over direct injection of an aqueous sample.
The extraction protocol shown here appears to be optimized for propylene glycol, as this compound exhibited a significantly better retention on ENVI-Carb Plus than ethylene glycol. Further investigation will be done to see if an alternative protocol can improve retention of ethylene glycol.
References
To continue reading please sign in or create an account.
Don't Have An Account?