Determination of Hydrocortisone from Topical Cream Using Discovery DSC-Si SPE and Reversed-Phase HPLC-UV
Hydrocortisone (Figure A) is a steroid hormone secreted by the adrenal cortex and synthesized for medicinal use. It is a moderately polar compound that contains a glucocorticoid structure (steroid nucleus), and is used to treat a variety of inflammatory and non-inflammatory conditions. Formulated in pharmaceutical-grade topical creams, it is often used to clinically reduce itching/pain caused by rashes.
In this study, we demonstrate the utility of Discovery SPE by developing a simple and selective normal-phase SPE assay which effectively isolates hydrocortisone from topical cream for subsequent reversed-phase HPLC-UV quantitation/analysis. Thin-Layer Chromatography (TLC) was used initially to determine appropriate load conditions, and recoveries from final SPE method conditions yielded recovery and RSD values of 99.86 ± 6.99%.

Figure A.Structure of Hydrocortisone
Problems Associated with Poor/No Sample Preparation
Most reversed-phase HPLC protocols call for an aqueous:organic mobile phase mixture to facilitate separation within the LC column. Although the national brand hydrocortisone cream sample (Hydrocortisone 1% Cream, Maximum Strength) tested within this report was soluble in methanol, when methanolic samples were exposed to buffer/water, precipitation occurred (Figure B). This was primarily due to the insolubility of lipophilic inactive ingredients within the topical cream. Such components include aloe barbadensis, cetearyl alcohol, glycerin, glyceral stearate, and a variety of oils.
As a result, a simple solvent extraction/dilution is inadequate sample preparation for this application. Lipophilic components within the cream can easily precipitate (upon exposure to the aqueous mobile phase) causing increased back pressure and system clogging.

Figure B.Precipitation of Lipophilic Components of Hydrocortisone Cream When Exposed to DI H2O:Methanol (1:1). (left vial- blank; right vial- precipitated sample)
Load Optimization Using TLC
Two separate Silica-60 TLC plates (2.5 x 7.5cm) were spotted with both a 1mg/mL methanolic hydrocortisone cream sample and hydrocortisone standard. Each plate was eluted separately with ethyl acetate or ethyl acetate:hexane (1:1). Spot migration occurred for both the standard and sample on the ethyl acetate TLC plate, whereas no spot migration occurred on the ethyl acetate:hexane TLC plate.
From the results above, hydrocortisone cream was dissolved in a ethyl acetate:hexane (1:2) prior to sample load on the Discovery DSC-Si (silica) SPE cartridge, 500mg/3mL (52654-U). The ethyl acetate component of the mixture was necessary to solubilize the topical cream. Hexane was needed to induce polar interaction required to adequately retain hydrocortisone on the silica SPE sorbent.
Excellent Sample Clean-Up, Recovery, & Reproducibility
Using the SPE method detailed in Table 1, six 1g aliquot samples of a national brand of hydrocortisone cream (Hydrocortisone 1% Cream, Maximum Strength) were extracted and analyzed using Discovery DSC-Si SPE cartridges (500mg/3mL) and Discovery HS C18 HPLC column, 5cm x 4.6mm, 5µm. As a result of the SPE procedure, lipophilic interferences inherent with the topical cream were sufficiently removed from the sample prior HPLC-UV analysis. This greatly reduced risks of sample precipitation upon contact with the mobile phase. This was further supported by the clean chromatograms observed during analysis (Figure C).
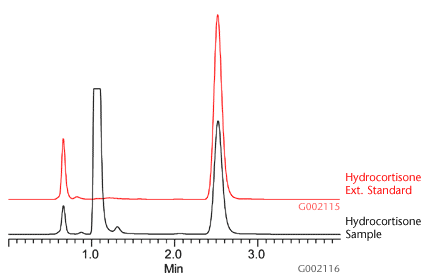
Figure C.Example Chromatogram of Hydrocortisone Extracts Using Discovery DSC-Si SPE
External standards were used to measure recovery against the known hydrocortisone weight-weight percentage described on the hydrocortisone cream manufacturer’s label. Using the SPE and HPLC method described in this report, an average absolute recovery and RSD value of 99.86 ± 6.99% (n=6) was observed to determine an average of 1.02% hydrocortisone in topical cream (Table 2).
Conclusion
Very often researchers are asked to isolate and quantitate a variety of compounds from difficult sample matrices. The SPE procedure described in this report offered an excellent means for separating the analyte of interest from key sample interferences prior to analysis. As a result, both the Discovery SPE and HPLC methods described in this report offered excellent accuracy and precision towards determining hydrocortisone levels in 1% hydrocortisone topical cream.
To continue reading please sign in or create an account.
Don't Have An Account?