Alkaline Phosphatase
Alkaline Phosphatase — Assays, Molecular Weight, Substrates, and Structure
We offer a broad range of alkaline phosphatase (ALP/ALKP) preparations optimized for conjugation to antibodies and other proteins for ELISA, Western blotting, and histochemical detection. Our BioUltra Grade Alkaline Phosphatase has a very high specific activity making it particularly useful for protein labeling when high sensitivity is required.
Western Blotting

Figure 1.FLAG-BAP control protein detected with anti-FLAG antibody and ALP-labeled secondary antibody and stained with BCIP/NBT substrate (using ProteoQuest Colorimetric Western Blotting Kit, B6404).
Immunohistochemistry
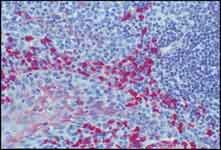
Figure 2.Formalin-fixed, paraffin-embedded section of human tonsil stained with Monoclonal Anti-Human IgA and an Anti-Mouse IgG ALP Conjugate using SIGMAFAST™ Fast Red TR/Naphthol AS-MX Tablets (Cat. No. F4523) as substrate. 40x
Our products are also used to dephosphorylate casein and other proteins. ALKP may be used to dephosphorylate the 5'-termini of DNA or RNA to prevent self-ligation. DNA or RNA can also be tagged with radiolabeled phosphate (via T4 polynucleotide kinase) after dephosphorylation with ALKP.
Bovine Alkaline Phosphatase
Bovine intestinal ALP is a dimeric, membrane-derived glycoprotein.1-3 At least three isoforms exist, which typically possess two N-linked and one or more O-linked glycans per monomer.2 The enzyme requires zinc, and magnesium or calcium divalent ions for activity.4
The enzyme has a broad specificity for phosphate esters of alcohols, amines, pyrophosphate, and phenols. It is routinely used to dephosphorylate proteins and nucleic acids.5-7
KM: 1.5 × 10–3 M (p-Nitrophenyl phosphate), 19 × 10–3 M (phosphoenolpyruvate)
Molecular weight:2,3 140–160 kDa (dimer MW)
E2781%: 7.6–10.5
Isoelectric point:4,8,9 Isozymes with a pI range of 4.4–5.8
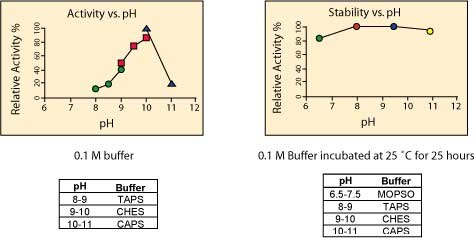
pH Optimum: The enzyme is most stable in the pH range 7.5–9.5.3 The pH optimum for enzymatic activity is pH 8-10. The pH optimum will change depending upon substrate, substrate concentration, and ionic concentration.8 The enzyme activity for this product is determined at pH 9.8 (diethanolamine buffer enzyme assay).
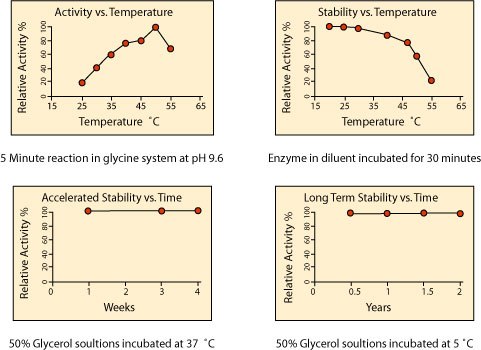
Temperature Effects on Activity and Stability
Inhibitors of Bovine Alkaline Phosphatase
Chelating agents, arsenate, cysteine, iodine, inorganic phosphate, pyrophosphate, diisopropyl phosphate, triphenylphosphate, diisopropyl fluorophosphate, and L-phenylalanine.9,10
Levamisole (Catalog Number L9756) is typically used to inhibit endogenous ALKP activity, while only slightly inhibiting the intestinal enzyme.11,12
Assay Protocols
Unit Definition: One DEA unit will hydrolyze 1 µmole of 4-nitrophenyl phosphate per minute at pH 9.8 at 37 °C. Approximately 3 DEA units equal one Glycine unit.
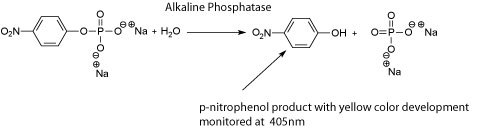
ALP is also used to determine the effectiveness of pasteurization on dairy products such as milk and cheese. ALP denatures when subjected to temperatures of approximately 72 °C for at least 15 seconds. Less extreme temperature conditions have been shown to destroy most milk-born pathogens. Therefore, ALP activity can be used to measure the effectiveness of pasteurization. In the past, two methods have been used to check for ALP activity after pasteurization, the first being the Scharer Test, which liberates and measures phenol. The second method is known as the Aschaffenberg & Mullen Test. This method measures the release of p-nitrophenol. With both tests negative results signify no ALP activity and the destruction of most milk-born pathogens. Recently new more sensitive techniques have been developed based on the same ALP activity principle.
References
E. coli Alkaline Phosphatase
E. coli ALKP is a dimeric, non-glycosylated protein assumed to reside mainly in the periplasmic space of E. coli. At least three isoforms exist. The enzyme requires zinc and is further activated by magnesium ions for activity.1-3 Like the bovine enzyme, the E. coli enzyme has a broad specificity for phosphate esters.1
KM: 0.02 × 10–3 M (p-Nitrophenyl phosphate)4
Molecular weight:2 89 kDa (Dimer MW)
E2781%: 7.6–10.52
Isoelectric point:5 Isozymes with a pI range of 4.5
pH Optimum: 8.05
Shrimp Alkaline Phosphatase
Shrimp ALKP is a dimeric protein with a molecular weight of approximately 58 kDa as determined by SDS-PAGE.
The Shrimp enzyme denatures at a lower temperature than other sources of the enzyme and is therefore recommended for dephosphorylation of DNA and RNA when a post-reaction heat inactivation step is required.
To continue reading please sign in or create an account.
Don't Have An Account?