Utilizing ISOGRO® Supplementation of M9 Minimal Media to Enhance Recombinant Protein Expression
Utilizing ISOGRO® Supplementation of M9 Minimal Media to Enhance Recombinant
Protein Expression
Stable isotope enrichment of recombinant proteins is necessary for most structure-function studies using NMR spectroscopy. The uniform incorporation of stable isotopes such as 13C, 15N, and D into proteins is routinely accomplished by using bacterial expression systems such as Escherichia coli (E. coli). Protein expression in E. coli typically involves the growth of bacteria in defined media where 13C and 15N containing glucose and ammonium salt are the sole sources of carbon and nitrogen. While bacterial protein production systems offer high cell mass and protein yields in rich media, the level of protein expression and cell mass obtainable in defined media is often lower. To compensate for reduced cell mass and recombinant protein yields, the large-scale production of protein in E. coli cells grown in M9 minimal or complex media is required which can be labor-intensive and expensive. Economical and efficacious production of adequate quantities of isotopically enriched protein for NMR experimentation is often the rate-limiting step in structural biology. Therefore, considerable interest exists in the development of protocols for the improvement of growth rates and recombinant protein expression levels in E. coli.
One strategy for the cost-effective optimization of protein expression in E. coli is the supplementation of M9 minimal media with ISOGRO®, an algal lysate-derived complex labeling medium. The inclusion of ISOGRO® in M9-based growth cultures provides the cells a metabolic boost that often decreases lag time, facilitates the attainment of growth saturation, and promotes recombinant protein production. The presence of algal cell lysate- derived complex growth media in cultures conserves cellular energy by limiting the requirement for de novo synthesis of cellular machinery and metabolic precursors. Minimizing energy expenditure permits more cellular resources to be direct towards recombinant protein expression. This protocol describes the usage of ISOGRO® as a supplement in minimal media to augment E. coli cell growth and protein production.
Advantages of Supplementation
- E. coli with as little as 10% ISOGRO® to M9
- Decrease lag time by as much as 60%
- Maximize OD and recombinant protein expression
- High level of recombinant protein expression with ISOGRO® supplement
Methods
Competent E. coli BL21(DE3) pLysS cells were freshly transformed with the expression plasmid containing the gene encoding for troponin C amino acid residues 1-89 (cTnC 1-89) controlled by the T7 promoter, spread aseptically on LB plates containing carbenicillin (Carb) and chloramphenical (Chl), and grown overnight at 37 °C. A single colony was grown 37 °C in LB with antibiotic selection until slightly turbid with shaking at 250 rpm. From this starter culture, 1mL was transferred to 50 mL of LB/Carb/Chl, grown at 37 °C with shaking, and the optical density was monitored at 600nm (OD600). The cells were harvested at OD600 ~ 0.9 by centrifugation and resuspended in 50 mL of sterile filtered minimal media (pH 7.0) containing 7 g/L Na2HPO4, 3 g/L KH2PO4, 2.5 g/L NaCl, 10.5 g/L K2HPO4, 0.5 g NaOH, 1 g/L 15NH4Cl (299251), 4 mM MgSO4, 10 μM FeCl3, 125 μM CaCl2, 50 μM ZnSO4, 2 g/L D-Glucose 13C6 (389374), 107 μg/L MgCl2•6H2O, 20 μg/L FeCl2•4H2O, 0.16 μg/L MnCl2•4H2O, 16 ng/L CuCl2•2H2O, 2.4 ng/L Na2MoO4•2H2O, 0.26 μg/L H3BO3, 1 mg/L choline chloride, 100 μg/L riboflavin, 50 mg/L niacin, 1 mg/L folic acid, 1 mg/L pyridoxal phosphate, 50 mg/L thiamine, 1 mg/L biotin, 1mg/L D-pantothenate. The minimal media starter culture was equally divided between 500 mL regular minimal medium and 500 mL of minimal media supplemented with 10% ISOGRO®-13C,15N powdered growth medium (606839) (0.5g ISOGRO® powder/500mL Medium) and grown at 37 °C with shaking. At an OD600 ~ 0.9, the protein expression was induced by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 0.1 mM. Protein production was analyzed by SDS-PAGE.
Results
Bacterial Growth Rates
Supplementation of minimal media with 10% ISOGRO® substantially reduced the lag time of cells by more than 60% as evidenced by the faster transition of cultures into log phase growth (Figure 1). Cells grown in ISOGRO® supplemented minimal media reached the target induction point of an OD600 ~ 0.9 in approximately 3 hours, versus 9 hours in standard M9 media. ISOGRO® is composed of an algal lysate containing metabolic precursors, which permits the cultures to initiate growth more rapidly, thus eliminating the need for de novo production of the various components of the metabolic pathways.
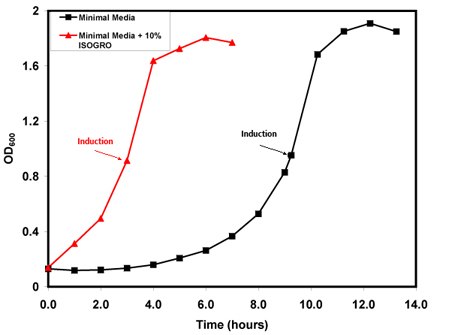
Figure 1.Growth Curve cTnC(1-89) pLysS. OD600 versus time (hours). Red curve is ISOGRO® supplemented minimal media and Black curve is minimal media alone.
Recombinant Protein Expression
The expression levels observed for cTnC(1-89) produced in the ISOGRO® enriched minimal media were greater compared to that obtained using unsupplemented minimal media (Figure 2). In the presence of 10% ISOGRO®, there was an increase in the amount of cTnC(1-89) expressed while the contaminating host cellular proteins were produced at a level comparable to that seen in uninduced minimal media and induced minimal media cultures. This observation is important in that subsequent recombinant protein purification is often facilitated when the target protein constitutes a high percentage of the total cellular protein.
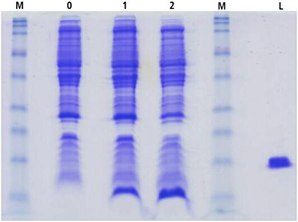
Figure 2.SDS-PAGE cTnC(1-89) cell lysates. M-low molecular weight maker; 0=uninduced; 1=induced minimal media; 2=induced minimal media + ISOGRO®; L=Lysozyme.
Discussion
Modern NMR techniques require the routine production of milligram quantities of uniformLy enriched recombinant protein. It is desirable to develop protocols for the economic and time-efficient isotopic enrichment of target proteins. The methodology presented here offers researchers the potential to reduce the labor hours and isotope expense by utilizing ISOGRO® supplementation of minimal media for recombinant protein expression. The inclusion of 10% ISOGRO® (0.5g/500mL of minimal media) in minimal media reduces the lag time of cultures and promotes the enhancement of target protein levels. It should be noted that further optimization of this protocol is possible by altering variables such as IPTG concentration, induction optical density, and percentage of ISOGRO® supplementation.
For more information on ISOGRO® or to inquire about receiving a free ISOGRO® sample for evaluation, please contact us.
Acknowledgements: Data generously provided by Dr. Paul R. Rosevear, The Department of Molecular Genetics, Biochemistry and Microbiology, University of Cincinnati Medical Center, Cincinnati, OH.
Products of Interest
To continue reading please sign in or create an account.
Don't Have An Account?